Clinical trials reporting platform
An expanding biotech company needed a secure and compliant platform to store, process, and analyze clinical trial data while meeting regulatory requirements (GxP, HIPAA, ISO 27001). They also required seamless integration with existing systems and support for AI analytics.
Approach:
We implemented a compliant cloud infrastructure (AWS, Azure, or GCP) with Databricks’ Data LakeHouse, ensuring a single source of truth with a complete processing history. The system provided automated data workflows, an intuitive data management interface, and AI-ready environments for advanced analytics.
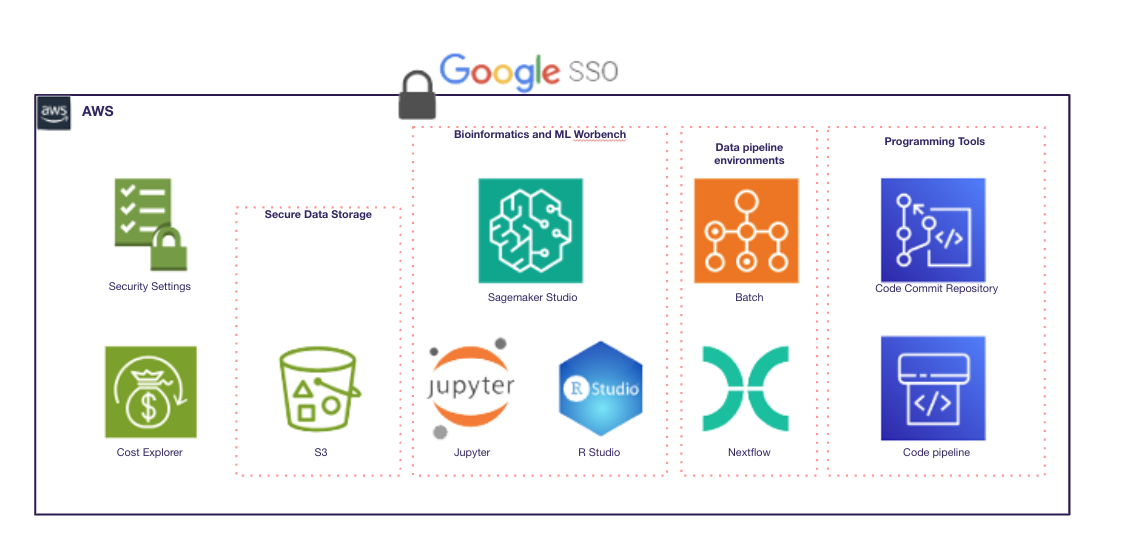
Results
Regulatory compliance with full data traceability.
User-friendly platform for scientists and analysts.
Seamless integration with existing tools, minimizing disruptions.
AI & machine learning support for advanced data insights.
This solution empowered the client with efficient data management, enabling faster decision-making and improved research outcomes.



