How a biotech company used the cloud and artificial intelligence to improve clinical data processing
In the biotech environment, effective management and analysis of clinical trial data is critical to regulatory compliance and scientific progress. A growing biotech company faced the challenge of storing, processing and analyzing massive amounts of clinical data while complying with strict regulatory standards. This case study explores how an artificial intelligence-based cloud solution enabled seamless data management, automated processing and enhanced analytics, ultimately supporting better decision-making and regulatory compliance.
Challenge
- The client needed a secure and compliant platform to store, process, and analyze clinical trial data.
- Legal and functional requirements, including compliance with GxP (21 CFR Part 11), HIPAA, HITRUST, ISO 27001, SOC 2 Type II, and UK Cyber Essentials Plus, had to be met.
- The system needed to support large-scale data processing for dashboards and AI/ML model training.
- Integration with existing workflows was essential to minimize disruption across multiple teams.
Approach
- Selected a compliant cloud provider (AWS, Azure, or GCP) to ensure security and scalability.
- Implemented a Data Lakehouse solution using Databricks for structured, semi-structured, and unstructured data storage and processing.
- Developed an intuitive user interface for managing data and processing workspaces.
- Integrated the system with existing infrastructure, ensuring minimal disruption while providing flexibility for data scientists.
- Enabled automated data processing to streamline workflows and enhance efficiency.
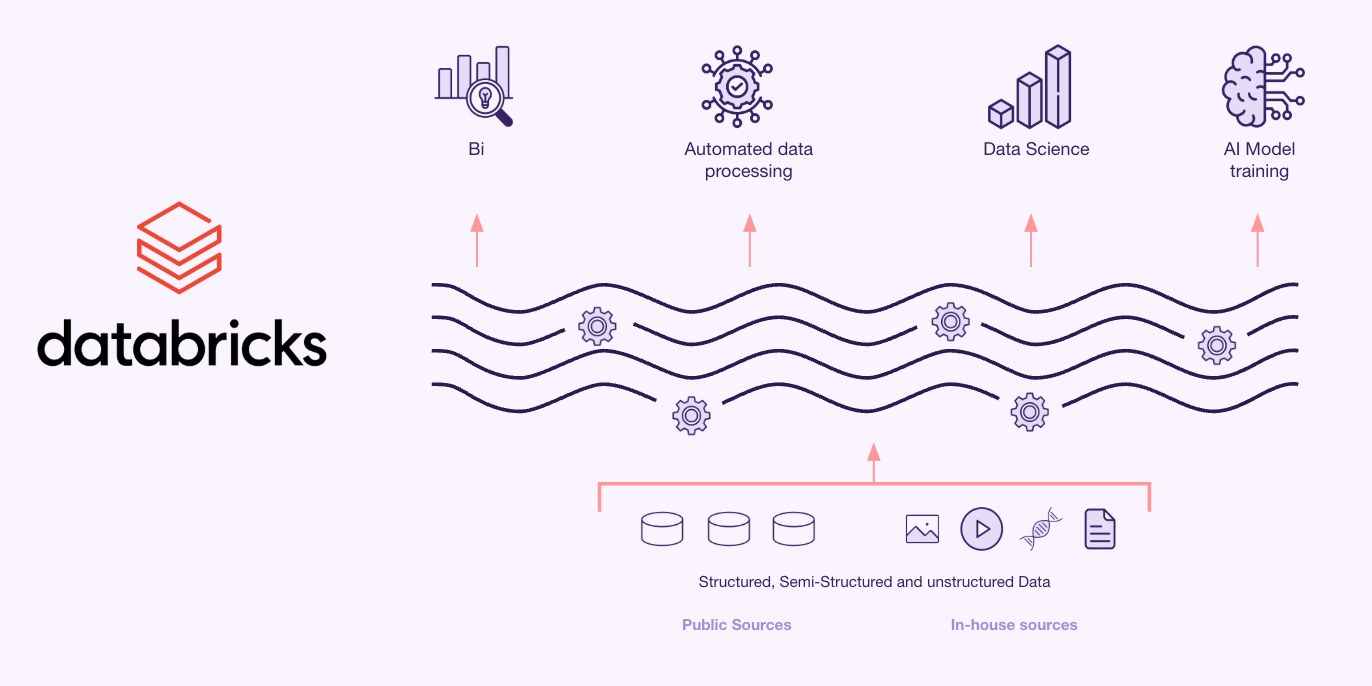
Results
- Single Source of Truth: A unified data platform with a full processing history for regulatory compliance.
- Enhanced Usability: Intuitive interface for seamless data management and analysis.
- Seamless Integration: The system integrated smoothly with existing tools, reducing workflow disruptions.
- Advanced Analytics: Enabled efficient AI/ML model training on clinical trial data.
Using a compliant cloud-based solution from Databricks, the client successfully transformed clinical trial reporting. The platform provided a secure, scalable and efficient way to process clinical data while ensuring compliance with stringent regulations. With automated data flows and seamless integration, the company can now focus on accelerating scientific discovery and regulatory submissions.



