In 1960, Klein et al. found that when mice developed primary chemically induced sarcomas, they also developed an anti-tumor immune response mediated by lymph node cells to a secondary challenge [1]. This discovery sparked an avalanche of studies that subsequently led to the development of the cancer immunotherapy field. In 1988, the first promising trials in patients with metastatic melanoma were carried out using T cells isolated from freshly resected melanoma and expanded in vitro [2]. Then, an adoptive transfer of anti-tumor specific T lymphocytes isolated from tumors (tumor-infiltrating lymphocytes, TILs) seemed to be an ideal candidate for killing tumor cells. The main advantages of TILs are their precision (only neoplastic cells are destroyed contrary to effects of chemotherapy and/or radiotherapy) and the killing mechanism – apoptosis (safer for the tissue, as opposed to necrosis). Unfortunately, further attempts to treat various types of cancers using TILs encountered many problems, mainly because of immunosuppressive tumor environment, but also due to a lack of standardization of this treatment method. The search for using autologous T cells with more predictable characteristics of tumor cell recognition had started.
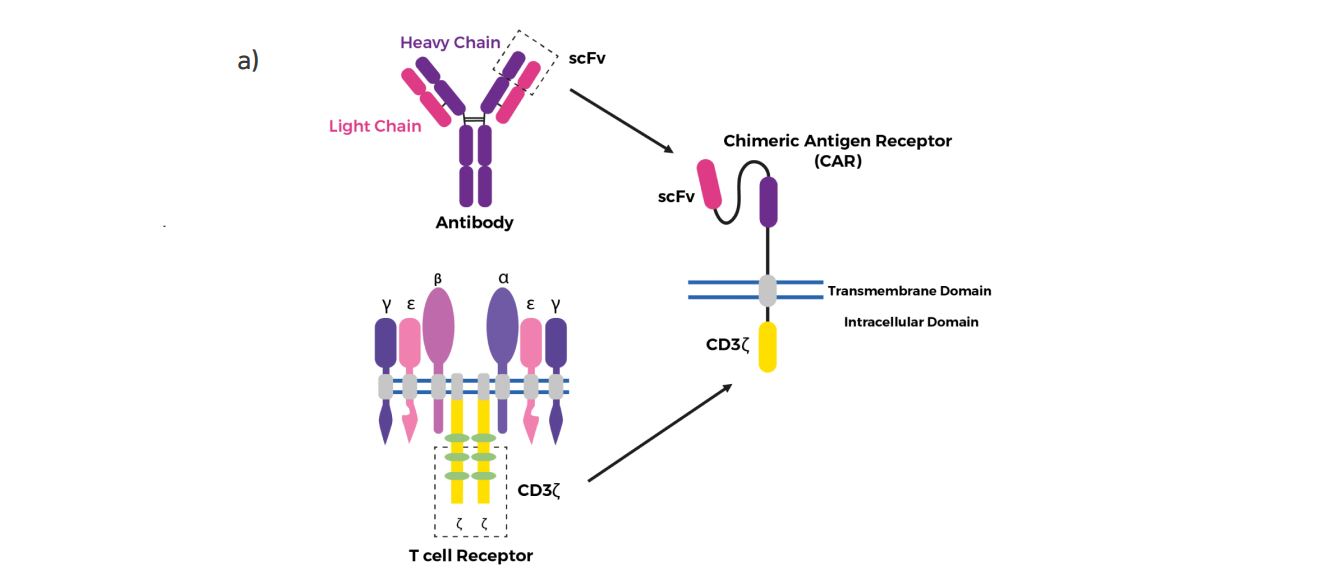
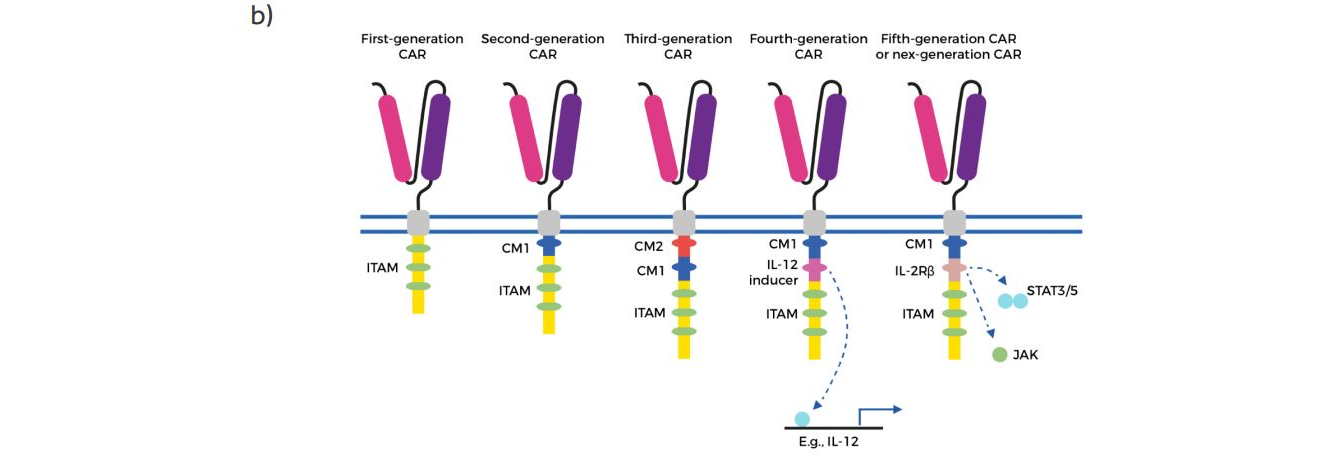
Figure 1. Structure of different chimeric antigen receptor (CAR) generations.
a) The core structure of a CAR, highlighting the major components of the extracellular domain, the
transmembrane domain and the intracellular domain (endodomain) – from the article The Evolving Protein Engineering in the Design of Chimeric Antigen Receptor T Cells, used under CC BY 4.0 / the colors have been changed from the original.
b) Evolution of the development of CARs from the first generation, which contained only ITAM motifs in the intracellular domain. Second-generation CARs included one co-stimulatory molecule (CM)1, and third-generation CARs contained a second CM. The fourth generation of CARs was based on second-generation CARs (containing 1–3 ITAMs) paired with a constitutively or inducibly expressed chemokine (e.g. IL-12). These T cells are also referred to as T cell redirected for universal cytokine-mediated killing (TRUCKs). The fifth, or ‘next generation’, is also based on the second generation of CARs, with the addition of intracellular domains of cytokine receptors (e.g. IL-2Rß chain fragment). ITAM immunoreceptor tyrosine-based activation motifs, CD co-stimulatory domain, IL-12 activation of interleukin 12 transcription, IL-2Rß truncated intracellular interleukin 2ß chain receptor with a STAT3/5 binding motif – from the article Teaching an old dog new tricks: next-generation CAR T cells / the colors have been changed from the original.
Currently, a number of studies are conducted on the clinical application of genetically modified T cells isolated from the patient’s blood. T cells are modified using virus vectors (retroviruses or lentiviruses) or a transposon system (Sleeping Beauty) to introduce a modified T cell receptor (TCR) or a so-called chimeric antigen receptor (CAR). CAR is a synthetic construct that recognizes native cell surface antigens including carbohydrate, lipid, protein antigens in the absence of the major histocompatibility complex (MHC) [3].
CAR consists of three domains: the extracellular domain recognizing with high-affinity antigens on tumor cells (a part of antibody -scFv), the transmembrane domain responsible for anchoring the receptor in the plasma membrane, stabilization and signal transduction, and the intracellular domain controlling T cell activation (Figure 1a) [4].
The first attempts and constructs of such modular single-chain CARs were presented in the 1990s by Esshar et al. [5] before the role of the individual subunits involved in the activation of T lymphocytes was fully understood. The proposed combination of single-chain antibody fragments (scFv) and the cytoplasmic tail of the TCR-associated ? chain led to the creation of what we now know as the first generation of CARs. The first CARs did not include costimulatory domains to provide the second signal for full T cell activation. Therefore, the further evolution of CARs concerned the intracellular domain to obtain greater efficiency of action as well as a longer survival time of CAR-T cells after administration to the recipient [4]. The second generation of CAR was created on the basis of the first generation by adding to the intracellular domain a costimulatory domain 4-1BB (CD137) or CD28 responsible for the production of interleukin-2, which promotes T cell activation and prevents apoptosis [6].
Currently, the third generation of CARs enriched with another intracellular domain to increase cytokine production and killing ability is being tested in clinical trials in the following combinations: CD3?-CD28-OX40 or CD3?-CD28-CD137. This new generation of CARs equipped with anti-CD20 and anti-HER2 scFV fragments have been tested in the treatment of leukemia and colon cancer. Unfortunately, the results of the treatment did not show any significant differences compared to the use of the second generation [7-8].
However, due to the small number of studies, it is yet difficult to evaluate the therapeutic potential of the third generation of CARs.
Figure 2. White blood cells attacking a cancer cell.
The result of further research on CARs modifications is the design of the fourth generation containing the construct for IL-12 in the intracellular domain (Figure 1b). These CARs are known as TRUCKs – T cells redirected for universal cytokine-mediated killing. TRUCKs, by releasing IL-12 in the locality of the tumor can polarize the immune response of local T cells towards Th1. Moreover, the presence of IL-12 activates and attracts innate immune cells to eliminate antigen-negative tumor associated cells that create a microenvironment for tumor growth [9].
The fifth generation or ‘next generation’ of CARs is based on the second generation with the addition of intracellular domains of cytokine receptors for IL-2 (IL-2Rß) with a binding site for the transcription factor STAT3. The antigen-specific activation of this CAR construct effectively provides all three synergistic signals required physiologically to drive full T cell activation and proliferation [10].
Currently on the market we have three CAR-T cells therapies. In 2017, two CAR-T therapies were approved by the US Food and Drug Administration (FDA) with the use of the second generation anti-CD19 CAR-T cells [11-12]. Highly promising and effective results were obtained in the treatment of B cell malignancies, including diffuse large B cell lymphoma (DLBCL) and B cell-precursor acute lymphoblastic leukemia (ALL). These therapies are named accordingly KYMRIAH™ (tisagenlecleucel), made by Novartis and approved patients under age 25 with ALL and for adults with DLBCL, and YESCARTA™ (axicabtagene ciloleucel), made by Kite Pharma/Gilead and approved for adults with relapsed or refractory large B-cell non-Hodgkin’s lymphoma (RR DLBCL). The price tag on these one-time therapies is quite high and remains at $475,000 for KYMRIAH™ and $373,000 for YESCARTA™.
In 2020, the FDA approved a third immunotherapy based, as in earlier therapies, on CD19-directed genetically modified autologous T cells. This therapy named TECARTUS™ (brexucabtagene autoleucel) developed by Kite Pharma/Gilead is indicated for adult patients with relapsed or refractory mantle cell lymphoma (MCL). The cost of TECARTUS™ is estimated to be similar to YESCARTA™ for a one-time infusion. An independent nonprofit Institute for Clinical and Economic Review (ICER) compared Kymriah and Yescarta with chemotherapy, considering patient survival, quality of life, and healthcare costs over the lifetime of the patient. General conclusion was that CAR-T cell therapies, despite high price, are ‘cost-effective’ in treating cancer, primarily by extending the lives of some patients, much more than traditional chemotherapy (CAR-T Therapies: Final Evidence Report).
Clinical studies have shown that the efficacy of therapy with autologous CAR-T cells is much more effective if lymphodepletion is induced prior to administration. This approach is most commonly used with cyclophosphamide and/or fludarabine. Although it has many toxic side effects, provides expansion and persistence of CAR-T cells [11-12]. Additional side effects seen after infusion of CAR-T cells within days after administration are cytokine release syndrome and neurotoxicity, which can be fatal. Nonetheless recent clinical trials using anti-CD19 CAR-T cells in the treatment of patients with ALL have shown very high efficacy, with 81% of treated patients achieving complete remission with acceptable side effects.
This unquestionable success has prompted researchers to search for new applications for the CAR-T cells in the treatment of other types of hematological cancer like multiple myeloma [13], chronic lymphocytic leukemia [14] and acute myeloid leukemia [15].
Another potential application that is currently being tested for CAR-T cells are solid tumors. The therapies innovation to the treatment of solid tumors is essential as cancer is becoming the second most prevalent and frequent cause of death worldwide. Solid tumors, such as lung, colon, prostate and breast cancer, are the major contributors to cancer mortality rates due to the lack of effective therapies.
We all hope that further randomized clinical trials using particularly the new generations of CAR-T cells resistant to the immunosuppressive tumor environment may allow in the future to achieve effective methods in the fight against certain types of solid tumors.
Bibliography
[1] Klein G, et al. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960. 20:1561-72.
[2] Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988. 319(25):1676-80. doi: 10.1056/NEJM198812223192527.
[3] Houot R, et al. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res. 3(10):1115-22. doi: 10.1158/2326-6066.
[4] Zhang C, et al. Engineering CAR-T cells. Biomark Res. 2017. 5:22. doi: 10.1186/s40364-017-0102-y.
[5] Eshhar Z, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 90(2):720-4. doi: 10.1073/pnas.90.2.720.
[6] Guedan S, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018. 3(1):e96976. doi: 10.1172/jci.insight.96976.
[7] Till BG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 119(17):3940-50. doi: 10.1182/blood-2011-10-387969.
[8] Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010. 18(4):843-51. doi: 10.1038/mt.2010.24.
[9] Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015.15(8):1145-54. doi: 10.1517/14712598.2015.1046430.
[10] Kagoya Y, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018. 24(3):352-359. doi: 10.1038/nm.4478.
[11] Neelapu SS, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. 377(26):2531-2544. doi: 10.1056/NEJMoa1707447.
[12] Schuster SJ, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017. 377(26):2545-2554. doi: 10.1056/NEJMoa1708566.
[13] Yan Z, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019. 6(10):e521-e529. doi: 10.1016/S2352-3026(19)30115-2.
[14] Vitale C, Strati P. CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front Oncol. 2020. 10:849. doi: 10.3389/fonc.2020.00849.
[15] Baumeister SH, et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol Res. 2019. 7(1):100-112. doi: 10.1158/2326-6066.CIR-18-0307.




