In the first week of April, Ardigen attended the Bio-IT World Conference and Expo in Boston, a global gathering of thought leaders and researchers working at the intersection of life sciences, technology and bioinformatics. The 2025 conference was a record-breaking event, with more than 2800 attendees, 310+ speakers and over 150 exhibitors sharing breakthrough in the evolution of data-driven drug discovery, precision medicine, genomics, AI, and digital health.
This year, Ardigen also had exciting news to share: we’ve officially joined the Google Cloud Partner Advantage Program. This strategic step strengthens our AI capabilities for transforming healthcare and life sciences, and we’re thrilled to be partnering with a platform that shares our commitment to innovation.
Our team, represented by Lukasz Pawel Nowak, Livia, Nasim Jamali, Kathy Laska, Marek Kudla, Piotr Faba, and Barbara Wyroba, presented several posters highlighting real-world case studies from our ongoing work on topics that spanned enabling real-time analytics in clinical trials, applying large language models (LLMs) and knowledge graphs (KGs) for drug repurposing, transforming GWAS exploration, and scaling pipelines with Nextflow.
We wanted to share some of the impactful things that happened at Bio-IT along with our perspective on how we can best use biological data and AI to move science forward.


From data silos to open collaboration
Across the conference, a major theme echoed from both industry leaders and technologists: unlocking real impact from AI in life sciences requires integrated, multimodal data and infrastructure that supports scientific agility. In the second day plenary session introduction, Jesse Cugliotta, Global Lead of Healthcare and Life Sciences at Snowflake, described the “8 Critical Pillars for Your Enterprise AI Strategy”
Cugliotta advocated for data architectures that can adapt to constant change — because “there will always be another silo, and there will always be another large language model.” Static, all-in-one systems won’t cut it. Instead, real-time, cloud-native architectures that support live data sharing, on-demand scalability, and multi-language access (from Python and SQL to plain English) are becoming the new standard.
He also emphasized that successful AI strategies hinge on the ability to leverage all types of data, including unstructured formats like clinician notes, audio logs, contracts and real-world evidence, which make up an estimated 80% of enterprise data but often remain inaccessible to AI models. Additionally, he pointed out the importance of training AI models on the full spectrum of biological data, and not just the “success stories”.
The talk also highlighted the critical importance of governance and security as foundational pillars of these emerging technologies, especially under regulations like 21 CFR Part 11. Out-of-the-box capabilities like native anonymization, time-stamped data lineage, and secure access controls are essential to AI’s long-term viability in clinical and regulatory settings.
Finally, no matter how advanced the tools, the real innovation comes from people. Cugliotta argued for building a culture of data and AI literacy across organizations as a tool to empower experts to make well-informed decisions.
Scaling genomic medicine and redefining disease screening
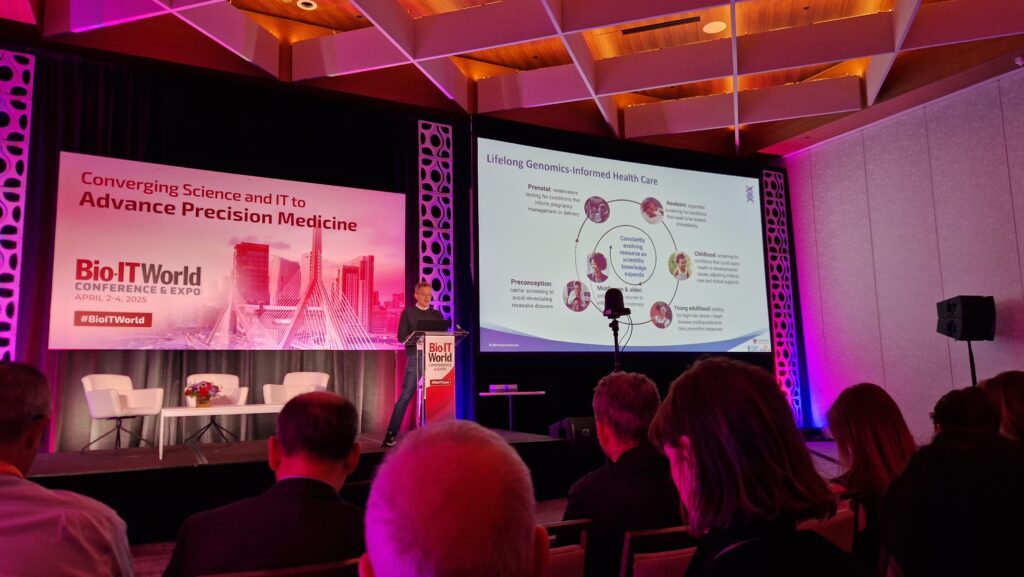
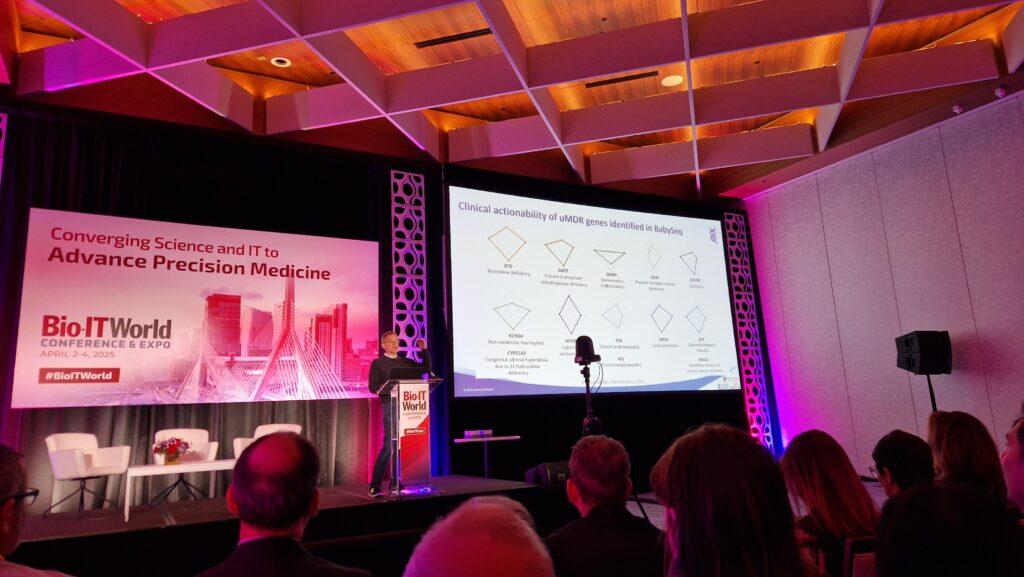
Among the many standout sessions was a keynote delivered by Dr. Robert Green, Profesor of Medicine at Harvard Medical School and the founder of Nurture Genomics. His talk, “Scaling Genomic Medicine: Transforming Newborn Screening through Informatics and Innovation,” offered a compelling look into how genomic data can reshape pediatric care.
Drawing on the BabySeq Project, which has led the way in sequencing newborns to detect actionable genetic conditions, Dr. Green illustrated both the promise and practical challenges of implementing genomic screening at scale. From ethical considerations and parental consent to informatics infrastructure and AI-driven decision support, the talk highlighted what it will take to bring precision medicine into early life.

Smarter omics pipelines with LLMs
In a standout talk, Dr. Weiwei Schultz of Johnson & Johnson Innovative Medicine shared how the company is harnessing large language models (LLMs) like GPT-4 to accelerate pipeline development within their Human Omics Hub. By integrating LLMs into bioinformatics workflows alongside tools like Nextflow for standardization and scalability, J&J has significantly reduced development time, improved documentation, and simplified onboarding for complex multi-omics analyses.



Key takeaways from Bio-IT - Ardigen’s perspective
From Ardigen’s perspective, Green’s and other sessions shared many common themes, which inspired some important insights:
Human-in-the-loop: As Dr. Weiwei Schultz’s presentation underscored, while LLMs can streamline logic interpretation, code generation, and documentation, human expertise remains essential to ensure scientific rigor and biological relevance. The future lies in combining intelligent automation with domain-specific fine-tuning to unlock faster, more reproducible insights in drug discovery.
Ethical and equitable implementation of genomic screening: As genomics moves into mainstream care, there’s a growing need for AI tools that make complex results interpretable for both clinicians and patients. Human-centric design and explainable AI will be crucial.
Learning from scalable genomic models: Dr. Green’s talk offered a glimpse into what’s possible when genomic medicine is embedded into national infrastructure. This scale is only achievable with robust, interoperable informatics and cloud-native, AI-ready pipelines that support longitudinal analysis across diverse cohorts.
Turning diagnoses into treatments: Where once a genetic diagnosis was an endpoint, today it’s increasingly a starting point for early intervention, particularly with gene therapies entering the clinic. Predictive models that align genomic markers with druggable pathways—or suggest candidates for repurposing—are becoming critical. This is where AI can reduce attrition and speed translation.
Collaboration as a catalyst for innovation: This point is not new, but it remains crucial: the next wave of genomic medicine will require tight collaboration across computational biology, clinical informatics and AI. It’s not just about sequencing more—it’s about understanding data faster and acting smarter.
For Ardigen, this is what we do best: whether it’s multiomic integration, data harmonization, or model development, we can help pharma and biotech partners extract true value from genomic data—and ultimately, turn insight into impact.
Driving impact
The 2025 Bio-IT World Conference was more than a showcase of innovation—it was a call to action. From scaling newborn screening to building future-proof AI architectures, progress in life sciences requires a culture of collaboration, critical thinking and data fluency across every part of the ecosystem.
This is our focus at Ardigen: helping partners turn fragmented data into actionable insights, and insights into impact. The 2025 Bio-IT World Conference was a reminder that what really drives innovation is not the tools—it’s in the people who use them.




