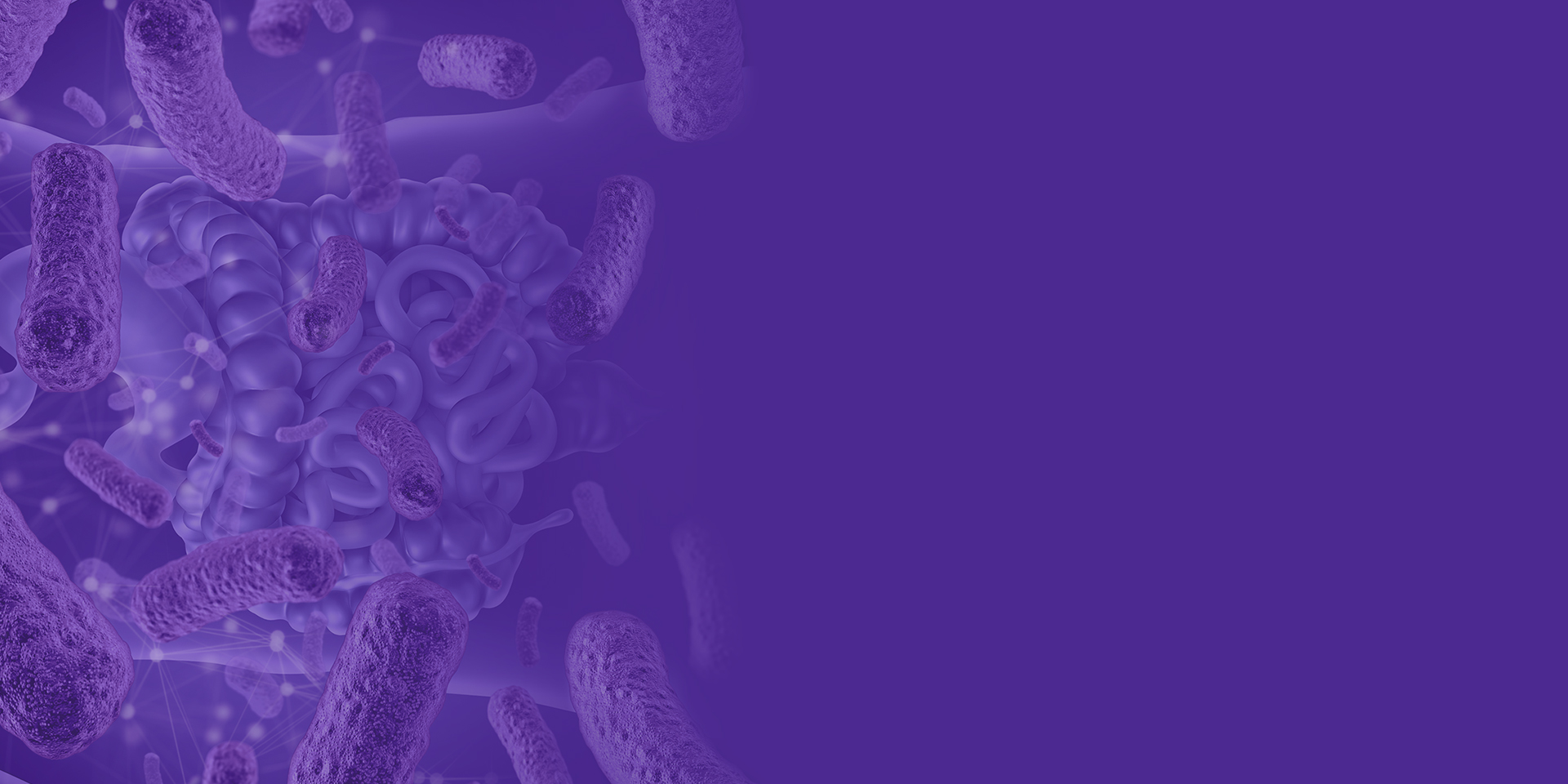
You have probably heard of the gut microbiome’s long-reaching arms which affect your bones, lungs, or even the brain. Yes, it turns out that microorganisms living inside our intestines can influence hematopoiesis, modulate pulmonary responses, or affect our emotions.
But how is this possible? How does the gut talk to those organs?
The key messengers in this network are microbial metabolites. Derived from the breakdown of nutrients, they are absorbed in the blood and reach distal body sites via circulation. In fact, the very nature of metabolites allows them to pass through even the toughest barriers, including the blood-brain barrier. But relax: over millions of years of evolution we have developed mechanisms to distinguish harmful metabolites, which are neutralized in the liver, from the good ones, which continue their journey.
Despite the staggering number of 172 million microbial protein sequences in the human gut [1], only 172 microbial metabolites have been identified so far [2]. Notably, even fewer have been tested for their immunomodulatory potential. Examples include short-chain fatty acids (derived from digestion of dietary fibers), indole derivatives (derived from metabolism of tryptophan), polyamines (derived from L-arginine), urolithin A (derived from polyphenolics), or lactic and pyruvic acids (products of sugar fermentation).
- Short-chain fatty acids (SCFA): mainly consisting of acetate, propionate and butyrate, SCFAs are best known for their anti-inflammatory properties. In experimental settings, they can protect mice against colitis [3], asthma [4] or intestinal infections [5]. Key to achieving this is the ability of SCFAs to influence the master orchestrators of the immune response, known as T helper cells. Butyrate can also act on cancer cells: it inhibits their proliferation and reduces tumor growth in mice. [6]. Interestingly, SCFAs can sometimes boost your immune system, providing evidence that metabolite function may be shaped by the immunological context. For example, in mice infected with influenza virus, SCFAs enhanced cytotoxic T cell response (a type of cells fighting off viruses) and contributed to faster viral clearance [7]. The main producers of SCFAs are bacteria belonging to Bacteroidetes and Firmicutes phyla. Bacteroidetes mainly produce acetate and propionate, while Firmicutes produce butyrate [8]. It is important to note that both of these phyla consist of many different species, each with different capacities to process fibers. Hence, it is the ultimate composition of the microbes that dictates the quantities and proportions of SCFAs, and thus their overall immunomodulatory potential.
- Indole derivatives are products of dietary tryptophan metabolism. They bind to a transcription factor important for the maintenance of epithelial barrier integrity. Their importance for intestinal homeostasis has been demonstrated in mouse models of colitis, candidiasis [9] and Citrobacter rodentium infection [10].
- Polyamines: derived from the metabolism of L-arginine, polyamines can enhance intestinal mucosal immunity. They have conferred several health benefits in experimental settings, e.g. protected mice against melanoma [11] or delayed senescence [12].
- Urolithin A: this microbial product is derived from metabolism of polyphenolics of berries and pomegranates. It has been shown to enhance gut barrier integrity and ameliorate colitis in mice [13].
- Pyruvic and lactic acid: these fermentation products of sugars enhanced immunity and survival after Salmonella Typhimurium infection in mice [14].
The examples described above highlight the enormous potential of microbial metabolites to improve human health. However, they constitute only the tip of the iceberg, and a range of new possibilities is becoming apparent. First, finding new metabolites with immunomodulatory properties and identifying their mechanisms of action will be the key to extending the utility of microbial metabolites for therapeutic purposes. Second, further optimization of the known metabolites in pre-clinical settings will facilitate their translation “from bench to bedside”.
But hold on! If these metabolites are derived from certain foods, wouldn’t it be simpler just to eat healthy foods? Could this offer a “one-size-fits-all” solution to improving human health? Unfortunately, it may not be so simple as it turns out that particular foods can be good for some people but bad for others. A “good vs bad” verdict can be based on a simple method involving the measurement of blood glucose spikes after eating (the higher the spike, the unhealthier the meal). Consumption of cookies or ice cream, for example, triggers different responses in different people, ranging from high spikes to almost no spikes [15] (YES! It means ice cream may not be bad for you).
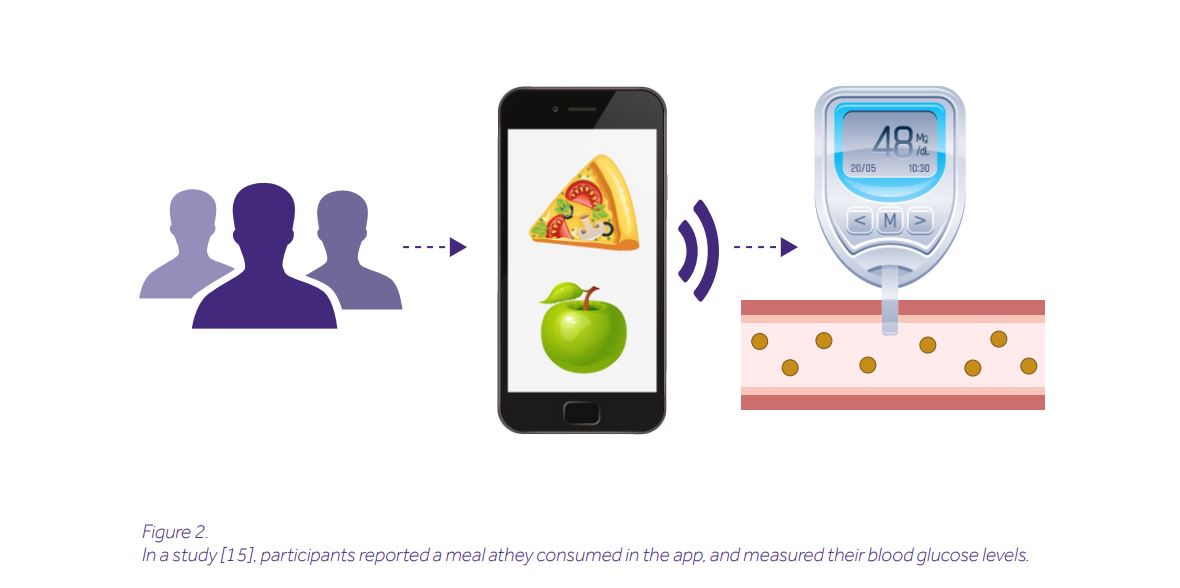
Why? The answer, again, is in the microbes that transform nutrients into metabolites. Thus, not diet alone but a diet personalized for your microbiome might constitute an alternative approach to the use of purified metabolites. Only if this is achieved, we can agree with Hippocrates’ quote: “Let food be thy medicine and medicine be thy food”.
Bibliography
[1] Almeida, A., et al., A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat Biotechnol, 2020.
[2] The Human Metabolome Database. 2020, October 08; Available from: https://hmdb.ca/.
[3] Furusawa, Y., et al., Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 2013. 504(7480): p. 446-50.
[4] Trompette, A., et al., Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med, 2014. 20(2): p. 159-66.
[5] Fukuda, S., et al., Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature, 2011. 469(7331): p. 543-7.
[6] Zagato, E., et al., Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol, 2020. 5(3): p. 511-524.
[7] Trompette, A., et al., Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity, 2018. 48(5): p. 992-1005 e8.
[8] Parada Venegas, D., et al., Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol, 2019. 10: p. 277.
[9] Zelante, T., et al., Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity, 2013. 39(2): p. 372-85.
[10] Kiss, E.A., et al., Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science, 2011. 334(6062): p. 1561-5.
[11] Geiger, R., et al., L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell, 2016. 167(3): p. 829-842 e13.
[12] Kibe, R., et al., Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep, 2014. 4: p. 4548.
[13] Singh, R., et al., Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun, 2019. 10(1): p. 89.
[14] Morita, N., et al., GPR31-dependent dendrite protrusion of intestinal CX3CR1(+) cells by bacterial metabolites. Nature, 2019. 566(7742): p. 110-114.
[15] Zeevi, D., et al., Personalized Nutrition by Prediction of Glycemic Responses. Cell, 2015. 163(5): p. 1079-1094.