Off-the-Shelf allogeneic cellular immunotherapies for cancer
As promised in our latest article “Current and future directions in CAR-T and TCR-T cell…”, which focused on therapies with the use of modified “classical” T cells against solid tumors, we are initiating a new series of articles that will explore novel approaches and their advantages in cellular immunotherapies. The high effectiveness of the adoptive immunotherapy in the treatment of human hematologic cancer has pushed current medicine into modern times. Adoptive cell transfer therapies are predominantly based on the transfer of autologous modified T cells. The reason behind sourcing T cells from the patient himself is to prevent the risk of adverse reactions like graft-versus-host disease (GvHD) and host allorejection. Unfortunately, the time needed for T cell isolation, their ex vivo modification and expansion may be too long, particularly for patients with rapidly progressive disease or those with an insufficient amount of the peripheral blood T cells [1]. Therefore, researchers have been exploring different allogeneic approaches for some time, examining their potential effectiveness and safety for the patient – which we would like to briefly cover.Different strategies for an off-the-shelf product
Rapid progress in genome modification has opened up new avenues in the design of cellular immunotherapies for cancer, including the development of “off-the-shelf” immune effector cells, transduced with a chimeric antigen receptor (CAR) or specific TCR construct (Fig. 1.). The development of a readily available source of effector cells derived from allogeneic donors is an attractive approach. However, the use of allogeneic cells carries the risk of GVHD and/or allorejection as a consequence of inappropriate matching of donor and recipient HLA molecules. Two main strategies are now being explored to avoid these adverse reactions and to develop a safe/“universal” source of allogeneic cell products: (i) genome editing of TCR and/or HLA class I expression in T cells and (ii) seeking for naturally occurring T cell subtypes or other populations across human lymphocytes that will not require genome editing for safe application.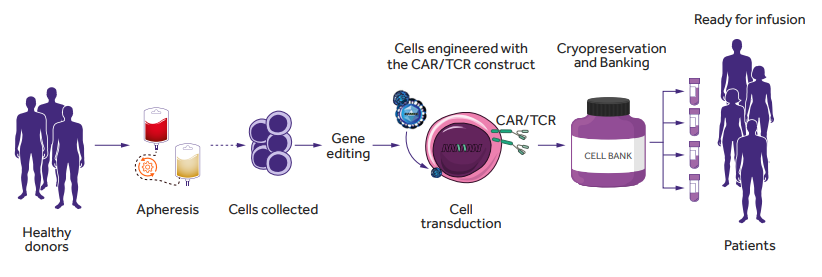
Figure 1. Allogeneic (“off-the-shelf”) CAR- or TCR-T cells generation. Allogeneic cells can be obtained from peripheral blood mononuclear cells from healthy donors. CAR- or TCR-T cells are generated by virus transduction and in vitro expansion before patient administration [2]. Figure based on the article: https://www.frontiersin.org/articles/10.3389/fimmu.2021.640082/full Used under CC BY-NC-ND 4.0
Towards universal T cell generation: preclinical outlook
Using allogeneic T cells in the adoptive immunotherapy carries the risk of GvHD as a consequence of donor TCRs recognizing the peptides presented by the host HLAs as antigenically foreign. On the other hand, the risk of allorejection is caused by the host TCRs recognizing donor pHLAs. These adverse reactions can be avoided by genetically impairing TCR and/or HLA class I expression in allogeneic T cells (Fig. 2). Disrupting the DNA sequences in the constant regions of ? or ß subunits of the TCR and/or HLA locus of MHC gene complex is achieved by using gene-editing techniques like: transcription activator-like effector nuclease (TALEN) [3-7] , zinc finger nuclease [8-10](, meganuclease [11] , megaTAL [12] and CRISPR/Cas9 system [13-15] . Because it is enough to target the T-cell receptor ?-chain constant (TRAC) locus to inactivate TCR function, at present, this approach is the most frequently used. It is important to note that the multiplex genome editing technique using the one-shot CRISPR system allows additional modifications (Fig. 2), such as loss of immune checkpoints, including programmed cell death 1 (PD-1) [14], or FasL or both PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression [16].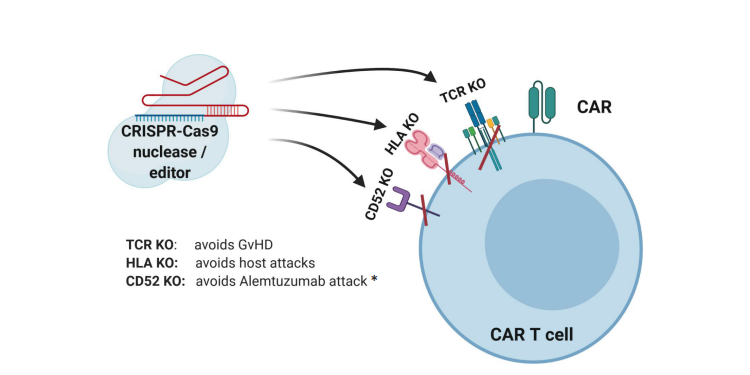
Figure 2. CRISPR-Cas9-mediated gene editing of CAR-T cells. The TCR can be knocked out to lessen the likelihood of graft versus host disease (GvHD). The HLAs can be knocked out to increase persistence of gene-modified cells. Knockout of receptors that can be targeted by other medications, such as antibodies, can be accomplished to allow selective survival of gene-modified cells, e.g., CD52 [17]. *Alemtuzumab is an anti-CD52 monoclonal antibody used to deplete host T cells prior to CAR-T cell therapy. It promotes survival and expansion of infused cells if donor CD52 is not present [18]. Figure based on the article: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01965/full Used under CC BY-NC-ND 4.0
All of the above approaches were carefully tested, mostly on animal models, and besides providing anti-leukemic effects, they were proven to reduce alloreactivity while improving resistance to spontaneous apoptosis and immunosuppression. The TALEN-modified cell therapy was tested in a small clinical trial in which two infants with relapsed refractory CD19+ B-cell acute lymphoblastic leukemia received allogeneic anti-CD19/TCR–/CD52– CAR-T cells. After a single-dose infusion of previously modified T cells, remission was achieved at 28 days in both infants, and CAR-T cells persisted until the health conditions of the children improved enough to allow successful allogeneic stem cell transplantation [5]. TALEN were also used to disrupt the TRAC locus to generate universal allogeneic CAR-T cells against the tumor-associated antigen CS1, which are currently tested in relapsed and refractory multiple myeloma patients (NCT04142619). In another ongoing clinical trial (NCT04150497), the TALEN method was used to generate universal anti-CD22 CAR-T cells to treat patients with relapsed or refractory B-cell acute lymphoblastic leukemia. The one-shot CRISPR-Cas9 (a revolutionary gene-editing technology) is another method being tested in ongoing clinical trials. Stadtmauer et al. (2020) demonstrated for the first time in a human clinical trial (NCT03399448) the safety and feasibility of this method in an autologous setting [19]. The potential therapeutic effect of allogeneic T cells modified by the CRISPR method is being currently tested in ongoing clinical trials in patients with B-cells leukemia and lymphoma (NCT03166878, NCT04035434), multiple myeloma (NCT04244656) and renal cell carcinoma (NCT04438083). All these studies, although at the beginning of development, are promising off-the-shelf therapies that avoid GvHD responses and improve the persistence of infused allogeneic T cells in the recipient.Naturally existing “universal” types of lymphocytes and preclinical outlook
An alternative approach to generate “off-the-shelf” products is to search for naturally existing immune cell types with a favorable safety profile. These “universal” donor immune cells do not induce GvHD, so they do not require extensive genome modification and have the potential to attack different types of tumor cells. Natural Killer (NK) cells are a perfect example, however, other T cell subtypes or populations, including virus specific memory T cells, CD1- and MR1-restricted T cells as well as ?? T cells are also promising cell types to be used in cancer immunotherapies.“Universality” of NK cells
As opposed to T cells, NK cells do not express TCR responsible for GvHD development and thus do not require TCR deletion [20]. In addition, high cytotoxic potential of NK cells against tumor cells goes along with the lack of IL-1 and IL-6 cytokines production, important mediators for cytokine-release syndrome [21, 22]. Unfortunately, the production of CAR-NK cells faces several practical problems compared to CAR-T cells, for example smaller NK cell numbers in donor blood, limited ex vivo expansion capacity, lower transduction efficiency, and loss of NK cell cytotoxic activity [2]. Despite these difficulties, in a recent clinical trial (NCT03056339), NK cells isolated from cord blood and modified in vitro into anti-CD19 CAR-NK cells, showed high efficiency in patients with hematological malignancies, (64% of complete responses) without manifestation of major toxic effects [23].“Universality” of antiviral T cells
Conventional ?ß-TCR T cells selected in the thymus are strongly restricted to self MHC and are a major pool of T cells circulating in the human body [24]. The polyclonality and a huge variety of TCR receptors allow T cells to recognize a wide repertoire of antigens that protect us not only from rapidly evolving pathogens, but also are responsible for maintaining tolerance. The TCR diversity is not constant during T cell differentiation and gets reduced upon antigen specific activation and clonal expansion such as in the course of T cell response to viral infection. The effectiveness of allogeneic memory virus-specific T cells has been proven in many clinical trials , and most importantly, it has been observed to have a very limited GvHD, which makes these cells a potential universal candidate for the cancer treatment [25]. Indeed, the use of memory virus-specific T cells as a universal starting platform for transduction of CAR or TCR is currently being investigated in phase I and II trials. For example, infusion of anti-CD19 CAR modified virus specific T cells in B-acute lymphoblastic leukemia patients led to cytotoxic elimination of B cells and complete remission in 2 out of 8 patients (NCT00840853)[26]. In a study on preventing acute myeloid leukemia recurrence in patients after allogeneic hematopoietic cell transplantation, the relapse-free survival was 100% at a median of 44 months (a control group patients had 54% relapse-free survival) following infusion of virus-specific T cells expressed a Wilms tumor antigen-1 specific TCR (NCT01640301) [27]. Which of the improvements in the treatment of hematological and non-haematological patients using allogeneic cells described here will find further practical application is difficult to predict, so please stay connected and follow our blog post on novel therapy types. In our next article, we will continue on the allogeneic approach and describe the potential use of other unconventional subsets of T cells, such as iNKT cells, MR1-restricted T cells (invariant mucosa-bound T cells) and ??-TCR T cells as “universal” donors in cancer immunotherapy.References:
- Boyiadzis MM, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018 Dec 4;6(1):137. doi: 10.1186/s40425-018-0460-5. PMID: 30514386; PMCID: PMC6278156.
- Martínez Bedoya D, et al. Allogeneic CAR T Cells: An Alternative to Overcome Challenges of CAR T Cell Therapy in Glioblastoma. Front Immunol. 2021 Mar 3;12:640082. doi: 10.3389/fimmu.2021.640082. PMID: 33746981; PMCID: PMC7966522.
- Poirot L, Philip B, et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75(18):3853–64.
- Valton J, et al. A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy. Mol Ther. 2015 Sep;23(9):1507-18. doi: 10.1038/mt.2015.104. Epub 2015 Jun 10. PMID: 26061646; PMCID: PMC4817890.
- Qasim W, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017 Jan 25;9(374):eaaj2013. doi: 10.1126/scitranslmed.aaj2013. Erratum in: Sci Transl Med. 2017 Feb 15;9(377):null. PMID: 28123068.
- Rasaiyaah J, et al. TCRαβ/CD3 disruption enables CD3-specific antileukemic T cell immunotherapy. JCI Insight. 2018 Jul 12;3(13):e99442. doi: 10.1172/jci.insight.99442. PMID: 29997304; PMCID: PMC6124532.
- Juillerat A, et al. Straightforward Generation of Ultrapure Off-the-Shelf Allogeneic CAR-T Cells. Front Bioeng Biotechnol. 2020 Jun 25;8:678. doi: 10.3389/fbioe.2020.00678. PMID: 32671047; PMCID: PMC7330105.
- Provasi E, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012 May;18(5):807-815. doi: 10.1038/nm.2700. PMID: 22466705; PMCID: PMC5019824.
- Torikai H, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–705.
- Torikai H, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122(8):1341–9.
- MacLeod DT, et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol Ther. 2017 Apr 5;25(4):949-961. doi: 10.1016/j.ymthe.2017.02.005. Epub 2017 Feb 23. PMID: 28237835; PMCID: PMC5383629.
- Hale M, et al. Homology-Directed Recombination for Enhanced Engineering of Chimeric Antigen Receptor T Cells. Mol Ther Methods Clin Dev. 2017 Jan 10;4:192-203. doi: 10.1016/j.omtm.2016.12.008. PMID: 28345004; PMCID: PMC5363294.
- Eyquem J, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017 Mar 2;543(7643):113-117. doi: 10.1038/nature21405. Epub 2017 Feb 22. PMID: 28225754; PMCID: PMC5558614.
- Ren J, et al. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin Cancer Res. 2017 May 1;23(9):2255-2266. doi: 10.1158/1078-0432.CCR-16-1300. Epub 2016 Nov 4. PMID: 27815355; PMCID: PMC5413401.
- Georgiadis C, et al. Long Terminal Repeat CRISPR-CAR-Coupled “Universal” T Cells Mediate Potent Anti-leukemic Effects. Mol Ther. 2018 May 2;26(5):1215-1227. doi: 10.1016/j.ymthe.2018.02.025. Epub 2018 Mar 6. PMID: 29605708; PMCID: PMC5993944.
- Ren J, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017 Mar 7;8(10):17002-17011. doi: 10.18632/oncotarget.15218. PMID: 28199983; PMCID: PMC5370017.
- Morgan MA, et al. Use of Cell and Genome Modification Technologies to Generate Improved “Off-the-Shelf” CAR T and CAR NK Cells. Front Immunol. 2020 Aug 7;11:1965. doi: 10.3389/fimmu.2020.01965. PMID: 32903482; PMCID: PMC7438733.
- Chakrabarti S, et al. Alemtuzumab (Campath-1H) in allogeneic stem cell transplantation: where do we go from here? Transplant Proc. 2004 Jun;36(5):1225-7. doi: 10.1016/j.transproceed.2004.05.067. PMID: 15251298.
- Stadtmauer EA, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020 Feb 28;367(6481):eaba7365. doi: 10.1126/science.aba7365. Epub 2020 Feb 6. PMID: 32029687.
- Pfefferle A, Huntington ND. You Have Got a Fast CAR: Chimeric Antigen Receptor NK Cells in Cancer Therapy. Cancers (Basel). 2020 Mar 17;12(3):706. doi: 10.3390/cancers12030706. PMID: 32192067; PMCID: PMC7140022.
- Veluchamy JP, et al. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front Immunol. 2017 May 31;8:631. doi: 10.3389/fimmu.2017.00631. PMID: 28620386; PMCID: PMC5450018.
- Shin MH, et al. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020 Mar 9;20(2):e14. doi: 10.4110/in.2020.20.e14. PMID: 32395366; PMCID: PMC7192832.
- Liu E, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020 Feb 6;382(6):545-553. doi: 10.1056/NEJMoa1910607. PMID: 32023374; PMCID: PMC7101242.
- Godfrey DI, et al. The burgeoning family of unconventional T cells. Nat Immunol. 2015 Nov;16(11):1114-23. doi: 10.1038/ni.3298. Erratum in: Nat Immunol. 2016 Feb;17(2):214. Erratum in: Nat Immunol. 2016 Apr;17(4):469. PMID: 26482978.
- Tzannou I, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2017 Nov 1;35(31):3547-3557. doi: 10.1200/JCO.2017.73.0655. Epub 2017 Aug 7. PMID: 28783452; PMCID: PMC5662844.
- Cruz CR, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013 Oct 24;122(17):2965-73. doi: 10.1182/blood-2013-06-506741. Epub 2013 Sep 12. Erratum in: Blood. 2014 May 22;123(21):3364. PMID: 24030379; PMCID: PMC3811171.
- Chapuis AG, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019 Jul;25(7):1064-1072. doi: 10.1038/s41591-019-0472-9. Epub 2019 Jun 24. PMID: 31235963; PMCID: PMC6982533.




