In our recent blog article titled, “CAR-T cell therapy: possible side effects and their clinical management”, we described the side effects associated with CAR-T cell therapies for cancer treatment and the standard procedures used to mitigate their impact. For the record, cytokine release syndrome (CRS) and complications from the nervous system are common toxic effects. In some extreme cases, treatment requires high doses and longer administration of corticosteroids, which of course makes the treatment process longer and more expensive. Since the mechanisms at the basis of these adverse effects are becoming more and more understood, researchers are wondering how to improve CAR-T cells. In this article, we will discuss the latest methods to improve the performance of CAR-T cells in terms of reducing on target/off tumor toxicity, preventing tumor escape, diminishing toxic side effects and the idea of an off-the-shelf product.
On target/off tumor toxicity – a problem that can be avoided.
CAR-T cells recognize tumor-associated antigens (TAA) that may be also present on healthy tissues, leading to so-called on-target/off-tumor toxicity (Hinrichs 2013). The identification of the right TAA is a holy grail in developing CAR-T and TCR-T therapies that is crucial in minimizing off-tumor side effects.
In the absence of truly specific tumor antigens, alternative strategies are explored to increase tumor specificity and avoid off-tumor toxicity. In one of them, CAR-T cells are only activated if tumor cells express at least two TAAs. This strategy was applied in a system that uses a synthetic Notch receptor (synNotch). When synNotch binds its ligand, the expression of the receptor for another TAA is activated. Only when the second TAA is recognized, the SynNotch CAR-T cell acts against tumor cells (Royba 2016, Moghimi 2021).
Another idea studied to minimize the destruction of healthy cells, while simultaneously maintaining high efficiency in killing cancer cells, is the use of inhibitory CAR-T cells. These engineered T cells express two essential receptors. The first one allows the recognition of target TAAs on cancer cells (triggering activation and killing). Whereas the second one binds with epitopes presented on normal tissues and contains domains from programmed cell death protein 1 (PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (inhibition of activation) (Fedorov 2013). In this case, normal cells that have the appropriate protein on their surface are not killed.
In studies on anti-CD19-CAR-T cells containing killer-cell immunoglobulin-like receptor (KIR)/PD-1 inhibitory receptors, modified T cells eradicate CD19+ malignant B cells, but do not affect normal B cells that are also CD19 positive, showing that anti-CD19/KIR CAR-T cells can be a promising strategy for preventing B cell aplasia observed in CD19-CAR-T cell therapy (Tao 2020).
Avoiding the tumor escape phenomenon.
Cancer cells have a well-known ability to evade immune response or treatment, by using processes known as tumor escape mechanisms (Rodriguez-Garcia 2020). The use of CAR-T cells expressing two receptors was also explored in diminishing the risk of tumor escape. Initial effectiveness of this strategy was demonstrated by in vivo experiments and clinical trials with CAR-T cells transfected with two chimeric receptors against CD19 and CD20 (Zah 2016, Shah 2020, Tong 2020). The presented results showed great potential in cases of antigen escape in anti-CD19 CAR-T cells monotherapy in B-cell malignancies. In other clinical studies, double positive CD19/CD22 CAR-T cells were tested in a small number of patients to evaluate efficacy and possible adverse effects. This study demonstrated high efficacy of such CD19/CD22 CAR bispecific T cells in inducing remission in adult patients with relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL), while retaining safety comparable to that of FDA approved monotherapies (Dai 2020).
These treatments were developed mainly to increase tumor specificity, but they perfectly illustrate how the problem of cancer cell evasion from immune surveillance can be resolved. In the described trials, the expression level of CD19 antigen was lowered or even lost on cancer cells, which normally would end up with unsuccessful therapy or relapse.
Diminishing and avoiding toxic side effects.
For optimal effectiveness and safety, CAR-T cells should promptly reduce their cytotoxic activity after clearing their cancer cell targets. In most cases, the immune system responds to the presence of foreign antigens using mechanisms that are proportional to the type of pathogen. Sometimes, even in a healthy individual, when the immune system is over-activated, healthy tissues can be damaged. This in turn may lead to the dysfunction of key organs, such as lung damage caused by a COVID-19 infection.
The meticulous immune-regulation of excessive cellular activity is the key to avoiding damage to own tissues. Therefore, scientists are trying to implement a switch-off mechanism in CAR-T cells to be able to terminate an unfavorable immune response.
One strategy is to control the survival time of activated CAR-T cells in the recipient’s body, by the use of CAR-T cells that express inducible caspase 9 (iCasp9) (Diaconu 2017) or herpes simplex virus type 1 thymidine kinase (Murty 2020). Thanks to such modifications, CAR-T cells could be eliminated by administering a drug inducing immediate apoptosis, directly after killing tumor cells. The similar method of ablation of therapeutic CAR-T cells is a surface expression of marker antigen such as truncated human epidermal growth factor receptor (tEGFR) on these cells. CAR-T cells tagged with tEGFR can be depleted after the administration of anti EGFR antibodies like cetuximab in antibody-dependent cell-mediated cytotoxicity (ADCC), or complement-dependent cytotoxicity (CDC) manner (Koneru 2015, Paszkiewicz 2016). Additionally, marking CAR-T cells with tEGFR can be useful in monitoring the efficiency of the transduction and purification process (Taştan 2020).
Another very interesting idea is the concept of universal and programmable CAR-T cells (UniCAR-T cells). These cells are not directed to a cell’s surface tumor antigens and become active only in the presence of a short-lived target module (TM) (Fig. 1). Therefore, after degradation of TM, UniCAR-T cells will automatically be switched off, reducing adverse effects (Bachmann 2017, Cho 2018).
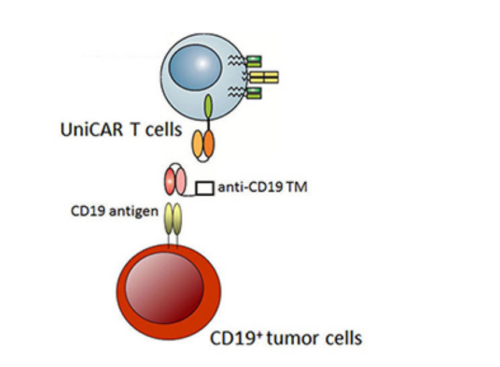 Figure 1. The concept of UniCAR-T cells.
Figure 1. The concept of UniCAR-T cells.
In the presence of target modules (TM) against the CD19 antigen, UniCAR-T cells are cross-linked to CD19+ tumor cells, which leads to their apoptosis. In the absence of the TM, UniCAR-T cells will automatically be switched off (Bachmann, et al; Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19
positive tumor cells. Oncotarget.2018; 9: 7487-7500.
Retrieved from https://www.oncotarget.com/article/23556/text/
used under CC BY 3.0
An elimination of modified T cells by apoptosis diminishes the toxicity, but also irreversibly ends the therapeutic effect. As an alternative, non-cytotoxic reversible methods of regulating the activity of CAR-T cells are also studied. The system of assembly of the active receptor recognizing TAAs uses one of them. The dimerization of the functional CAR is limited by the presence of a small molecule (Fig. 2) (Wu 2015, Mata 2017).
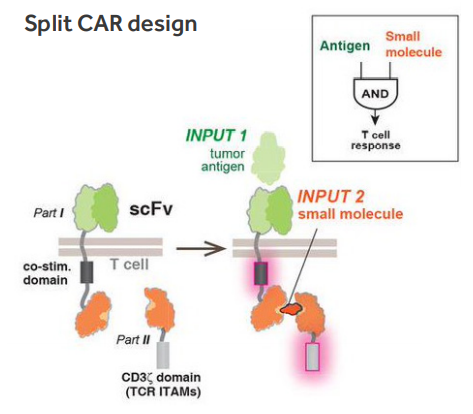 Figure. 2. Molecular strategies to control T cell activation (Wu 2015).
Figure. 2. Molecular strategies to control T cell activation (Wu 2015).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4721629/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4721629/bin/nihms751362f1.jpg
The similar method of pharmacological regulation of CAR-T cells activity is a model where administration of antibiotics like doxycycline induce expression of fully functional CAR in recipients, enabling accurate control of their activation after infusion (Sakemura 2016, Gu 2018). This apparently safe mechanism also has limitations, like the relatively slow induction and/or decreased expression of receptors. However, residual CAR expression has been observed, even in the absence of an inducer (Sakemura 2016, Gu 2018). This type of dose-dependent pharmacological regulation could allow precise control of a treatment’s duration, location and level of CAR-T cells activity, thus reducing toxicity.
Allogeneic approach – the idea for off-the-shelf product.
Although approved autologous CAR-T therapies work well without inducing graft-versus-host disease (GvHD), the time needed to prepare CAR-T cells from T cells that are isolated from patients is too long (about 2-6 weeks) and the disease may progress further before starting CAR-T treatment. In some cases, patients did not receive treatment due to the rapid disease progression (Schuster 2017, Maude 2018).
Therefore, an interesting solution seems to be the use of allogeneic T cells as an off-the-shelf product, which can be delivered to patients without manufacturing wait time. The use of allo-CAR-T cells carries the risk of developing dangerous alloreactivity, although clinical observations indicate that this is a relatively rare phenomenon. By 2018, from 132 patients with B cell malignancies treated with allo-CAR-T cells, 4% (n=5) and 3% (n=4) developed acute GvHD and chronic GvHD, respectively (Smith 2018).
To avoid GvHD, two approaches are currently being used: immunosuppression by administration of fludarabine and cyclophosphamide or alemtuzumab (anti-CD52 monoclonal antibody) prior to allo-CAR-T cells infusion (Qasim 2019) or depletion of TCR (Torikai 2012), unless the donor is a human leukocyte antigen (HLA) match. Another unfavorable situation is the reaction of the recipient lymphocytes against allo-CAR-T cells (host-versus-graft), therefore the inhibition of β2-microglobulin expression is investigated (McCreedy 2018).
A completely new approach widely studied recently is the use of gamma delta (γδ) T cells or natural killer (NK) cells as source of an off-the-shelf CAR modified cells. A natural lack of classical HLA restriction makes these cells optimal candidates for allogeneic cell therapy (Fleischer 2019).
Production time shortened – Fast CARs.
CAR-T cell manufacturing is a time-consuming process, e.g. in phase 2 clinical trials for Yescarta, it took 14 – 51 days from T cell collection to delivery of a product (https://www.fda.gov/media/108788/download). Some of the patients cannot wait that long and researchers are working on speeding up the production process. Recently, Gracell Biotechnologies received IND approval from China NMPA for its FasTCAR therapy for the treatment of relapsed or refractory adult B-ALL with the possibility of preparing autologous CAR-T cells in just two days (https://www.prnewswire.com/news-releases/gracell-biotechnologies-receives-ind-approval-from-china-nmpa-for-gc019f-a-fastcar-enabled-car-t-therapy-for-the-treatment-of-relapsed-or-refractory-adult-b-all-301209901.html) (Fig. 3).
Which of the improvements in cancer treatment using CAR-T cells described here will find wide application is difficult to predict today. Molecular biology is constantly moving forward, giving researchers tools to better understand the complex biology of cancer, as well as providing tools to design innovative cell therapies.
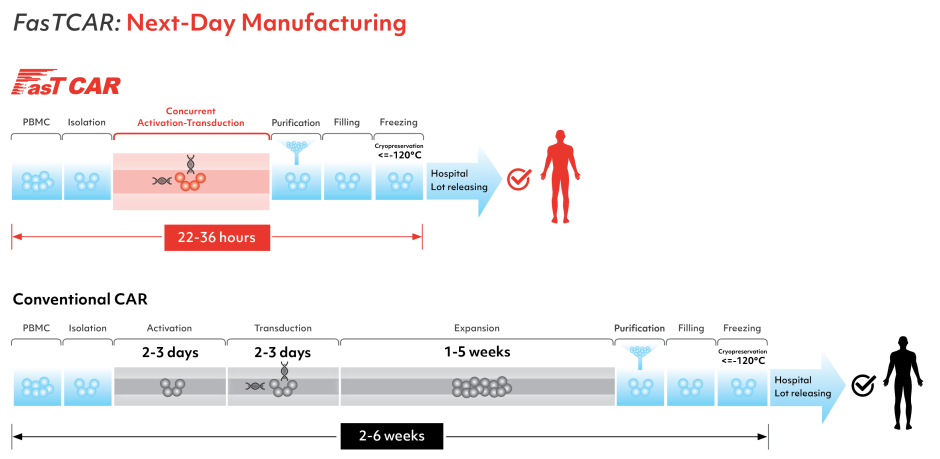
Figure 3. Comparison of the current production method of CAR-T cells with the FastCAR system.
https://www.gracellbio.com/fastcar/
https://www.gracellbio.com/img/Gracell_Workflow_FasTCAR.png)
In the next upcoming blog article, we would like to switch gears and introduce our readers to TCR-T based therapies, to which we would like to kindly invite you to read.
References:
Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013 Nov;31(11):999-1008. doi: 10.1038/nbt.2725. Epub 2013 Oct 20. PMID: 24142051; PMCID: PMC4280065.
Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016 Feb 11;164(4):770-9. doi: 10.1016/j.cell.2016.01.011. Epub 2016 Jan 28. PMID: 26830879; PMCID: PMC4752902.
Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, Hogarty MD, Barrett DM, Shimada H, Asgharzadeh S. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021 Jan 21;12(1):511. doi: 10.1038/s41467-020-20785-x. PMID: 33479234; PMCID: PMC7820416.
Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013 Dec 11;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. PMID: 24337479; PMCID: PMC4238416.
Tao L, Farooq MA, Gao Y, Zhang L, Niu C, Ajmal I, Zhou Y, He C, Zhao G, Yao J, Liu M, Jiang W. CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19+HLA-C1– Malignant B Cells While Sparing CD19+HLA-C1+ Healthy B Cells. Cancers (Basel). 2020 Sep 13;12(9):2612. doi: 10.3390/cancers12092612. PMID: 32933182; PMCID: PMC7564565.
Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ Jr, Guedan S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front Immunol. 2020 Jun 17;11:1109. doi: 10.3389/fimmu.2020.01109. PMID: 32625204; PMCID: PMC7311654.
Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016 Jun;4(6):498-508. doi: 10.1158/2326-6066.CIR-15-0231. Epub 2016 Apr 8. PMID: 27059623; PMCID: PMC4933590.
Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, Han X, Ti D, Dai H, Wang C, Yang Q, Liu W, Wang Y, Wu Z, Han W. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020 Oct 1;136(14):1632-1644. doi: 10.1182/blood.2020005278. PMID: 32556247; PMCID: PMC7596761.
Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, Cunningham A, Hamadani M, Fenske TS, Dropulić B, Orentas R, Hari P. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020 Oct;26(10):1569-1575. doi: 10.1038/s41591-020-1081-3. Epub 2020 Oct 5. PMID: 33020647.
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, Zhang Y, Chen M, Yang Q, Wang Y, Han W. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020 Apr 3;13(1):30. doi: 10.1186/s13045-020-00856-8. Erratum in: J Hematol Oncol. 2020 May 18;13(1):53. PMID: 32245502; PMCID: PMC7126394.
Diaconu I, Ballard B, Zhang M, Chen Y, West J, Dotti G, Savoldo B. Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Mol Ther. 2017 Mar 1;25(3):580-592. doi: 10.1016/j.ymthe.2017.01.011. Epub 2017 Feb 8. PMID: 28187946; PMCID: PMC5363196.
Murty S, Labanieh L, Murty T, Gowrishankar G, Haywood T, Alam IS, Beinat C, Robinson E, Aalipour A, Klysz DD, Cochran JR, Majzner RG, Mackall CL, Gambhir SS. PET Reporter Gene Imaging and Ganciclovir-Mediated Ablation of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2020 Nov 1;80(21):4731-4740. doi: 10.1158/0008-5472.CAN-19-3579. Epub 2020 Sep 21. PMID: 32958548.
Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015 Mar 28;13:102. doi: 10.1186/s12967-015-0460-x. PMID: 25890361; PMCID: PMC4438636.
Paszkiewicz PJ, Fräßle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, Busch DH. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016 Nov 1;126(11):4262-4272. doi: 10.1172/JCI84813. Epub 2016 Oct 17. PMID: 27760047; PMCID: PMC5096899.
Taştan C, Kançağı DD, Turan RD, Yurtsever B, Çakırsoy D, Abanuz S, Yılancı M, Seyis U, Özer S, Mert S, Kayhan CK, Tokat F, Açıkel Elmas M, Birdoğan S, Arbak S, Yalçın K, Sezgin A, Kızılkılıç E, Hemşinlioğlu C, İnce Ü, Ratip S, Ovalı E. Preclinical Assessment of Efficacy and Safety Analysis of CAR-T Cells (ISIKOK-19) Targeting CD19-Expressing B-Cells for the First Turkish Academic Clinical Trial with Relapsed/Refractory ALL and NHL Patients. Turk J Haematol. 2020 Nov 19;37(4):234-247. doi: 10.4274/tjh.galenos.2020.2020.0070. Epub 2020 Aug 4. PMID: 32755128; PMCID: PMC7702660.
Bachmann D, Aliperta R, Bergmann R, Feldmann A, Koristka S, Arndt C, Loff S, Welzel P, Albert S, Kegler A, Ehninger A, Cartellieri M, Ehninger G, Bornhäuser M, von Bonin M, Werner C, Pietzsch J, Steinbach J, Bachmann M. Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19 positive tumor cells. Oncotarget. 2017 Dec 21;9(7):7487-7500. doi: 10.18632/oncotarget.23556. PMID: 29484126; PMCID: PMC5800918.
Cho JH, Collins JJ, Wong WW. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell. 2018 May 31;173(6):1426-1438.e11. doi: 10.1016/j.cell.2018.03.038. Epub 2018 Apr 26. PMID: 29706540; PMCID: PMC5984158.
Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015 Oct 16;350(6258):aab4077. doi: 10.1126/science.aab4077. Epub 2015 Sep 24. PMID: 26405231; PMCID: PMC4721629.
Mata M, Gerken C, Nguyen P, Krenciute G, Spencer DM, Gottschalk S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017 Nov;7(11):1306-1319. doi: 10.1158/2159-8290.CD-17-0263. Epub 2017 Aug 11. PMID: 28801306; PMCID: PMC5780189.
Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, Koyama D, Goto T, Hanajiri R, Nishida T, Murata M, Kiyoi H. A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol Res. 2016 Aug;4(8):658-68. doi: 10.1158/2326-6066.CIR-16-0043. Epub 2016 Jun 21. PMID: 27329987.
Gu X, He D, Li C, Wang H, Yang G. Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety. Int J Mol Sci. 2018 Nov 3;19(11):3455. doi: 10.3390/ijms19113455. PMID: 30400287; PMCID: PMC6275001.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017 Dec 28;377(26):2545-2554. doi: 10.1056/NEJMoa1708566. Epub 2017 Dec 10. PMID: 29226764; PMCID: PMC5788566.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018 Feb 1;378(5):439-448. doi: 10.1056/NEJMoa1709866. PMID: 29385370; PMCID: PMC5996391.
Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood. 2018 Mar 8;131(10):1045-1052. doi: 10.1182/blood-2017-08-752121. Epub 2018 Jan 22. PMID: 29358181; PMCID: PMC5865610.
Qasim W. Allogeneic CAR T cell therapies for leukemia. Am J Hematol. 2019 May;94(S1):S50-S54. doi: 10.1002/ajh.25399. Epub 2019 Feb 1. PMID: 30632623.
Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovich B, Lee DA, Champlin RE, Bonini C, Naldini L, Rebar EJ, Gregory PD, Holmes MC, Cooper LJ. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012 Jun 14;119(24):5697-705. doi: 10.1182/blood-2012-01-405365. Epub 2012 Apr 24. Erratum in: Blood. 2015 Nov 26;126(22):2527. Rabinovitch, Brian [corrected to Rabinovich, Brian]. PMID: 22535661; PMCID: PMC3382929.
McCreedy BJ, Senyukov VV, Nguyen KT. Off the shelf T cell therapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018 Jun;31(2):166-175. doi: 10.1016/j.beha.2018.03.001. Epub 2018 Mar 28. PMID: 29909917.
Fleischer LC, Spencer HT, Raikar SS. Targeting T cell malignancies using CAR-based immunotherapy: challenges and potential solutions. J Hematol Oncol. 2019 Dec 29;12(1):141. doi: 10.1186/s13045-019-0801-y. PMID: 31884955; PMCID: PMC6936092.




