Cytokine release syndrome (CRS)
The antigen recognition by CAR-T cells leads to their activation. As a result, it causes the rapid proliferation of CAR-T cells, massive cytokine production and induction of apoptosis of target cells. The release of high amounts of cytokines promotes deterioration of tumor cells, but also activates yet more white blood cells that release cytokines, which is known as CRS. Among others, interferon gamma (IFN-?), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL) 10 and IL-6 are produced. The common clinical symptoms of CRS are fever, nausea, anorexia, renal or cardiac dysfunction, tachycardia, hypotension and hepatic failure [3]. The severity of the patient’s response to the massive production of pro-inflammatory cytokines varies greatly and depends on many factors. In clinical trials, 4 grades of CRS are distinguished. The first 2 grades correspond to mild symptoms whereas the grades 3 & 4 require additional treatment because they characterize life-threatening conditions [5]. In selected clinical trials for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia with CD19 CAR-T cells, the incidence of severe CRS (grade 3 or 4) has fluctuated between 13 and 45 percent [6, 7, 8]. Mild CRS develops in most patients treated with anti-CD19 CAR-T cells and no correlation was noted between the severity of side effects and the effectiveness of treatment. In this context, it seems extremely important to appropriately determine the grade of CRS at an early stage in order to select an effective method of inhibiting this uncontrolled inflammation without affecting the anti-cancer response. Currently, in order to counteract associated side effects (grade 2 or higher), physicians use a monoclonal antibody blocking IL-6 receptor (tocilizumab) and/or systemic administration of corticosteroids (e.g. dexamethasone). The use of dexamethasone or methylprednisolone, as shown by studies, quickly and effectively inhibits CRS, but generally does not affect the anti-cancer response. Only in high doses with prolonged use (over 2 weeks) it has shown the slight reduction of effectiveness of CAR-T cells [9]. Usually, a steroid treatment begins with the administration of small doses of dexamethasone (2–5 mg/dose) and if they do not bring the expected benefits, the dose is gradually increased up to 20 mg/m2 per day. Alternatively the usage of methylprednisolone at a dosage of 10 mg/kg per day can be proposed instead. If the therapy of CRS is successful, the dose may be reduced or even discontinued [10]. Furthermore, another common practice is the administration of tocilizumab (8 mg/kg every 8 hours as needed, no more than 3 doses in a 24 hour period or a total of 4 doses are recommended (Package Insert-YESCARTATM (fda.gov)). It can be applied both as an alternative of corticosteroids or in combination with them. Tocilizumab is less effective than corticosteroids, therefore usually is used in mild CRS conditions. Furthermore, it has been reported to reduce the amount of side effects without deteriorating the effectiveness of CAR-T cells [6, 11].Neurotoxicity (NTX)
NTX manifests itself with many neurological symptoms related to the changes in the white matter of the brain tissue. These include confusion, headache, lethargy, delirium, expressive aphasia, obtundation, myoclonus and seizure. The mechanism of damage is not fully known, but it seems that the main role is played by cytokines and/or immune effector cells crossing the blood-brain barrier [3, 4]. NTX usually occurs after the onset of CRS, and often after its resolution, importantly, it can rarely present in the absence of a previous CRS [6, 7, 5]. Most NTX is mild and self-limited, the time of occurrence varies within the range from 4-10 days after the infusion of CAR-T cells. The severity, kinetics and clinical symptoms of NTX may differ between those receiving different CAR-T cell products and now, with proper treatment in a majority of cases has been reversible [9, 5]. Detailed analysis of the cerebral spinal fluid from NTX patients has shown elevated levels of cytokines including interferon-?, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 (MCP-1/CCL2) and granzyme B [12, 13]. The median duration of neurological symptoms ranges from 5-14 days [7, 12, 13] and as it mostly takes place after the occurence of CRS, therefore usually patients are already hospitalized. Treatment methods of NTX vary from center to center and are based on clinical observations and depend on the symptoms severity. Neurotoxicity grading assessment is based on 4 grades as in case of CRS. Patients with grade 1 are usually admitted to the hospital for monitoring but drugs are often not administered. If symptoms worsen to grade 2, corticosteroids, like dexamethasone, that have good central nervous system (CNS) penetration are usually considered and are often selected as a first-line therapeutics for NTX treatment. With the higher severity of NTX symptoms (grade 3 and 4), the dose of corticosteroids is gradually increased (10 mg every 6 hours) or methylprednisolone is introduced instead/- concurrently (1 gram every 24 hours) and patients also require additional tests e.g. continuous EEG monitoring, head imaging to assess for abnormalities, such as edema or hemorrhage [5, 10]. In the absence of concomitant CRS the use of tocilizumab is generally not recommended in NTX treatment as it does not cross the blood-brain barrier and may paradoxically increase the concentration of IL-6 in the CNS [12, 14, 15].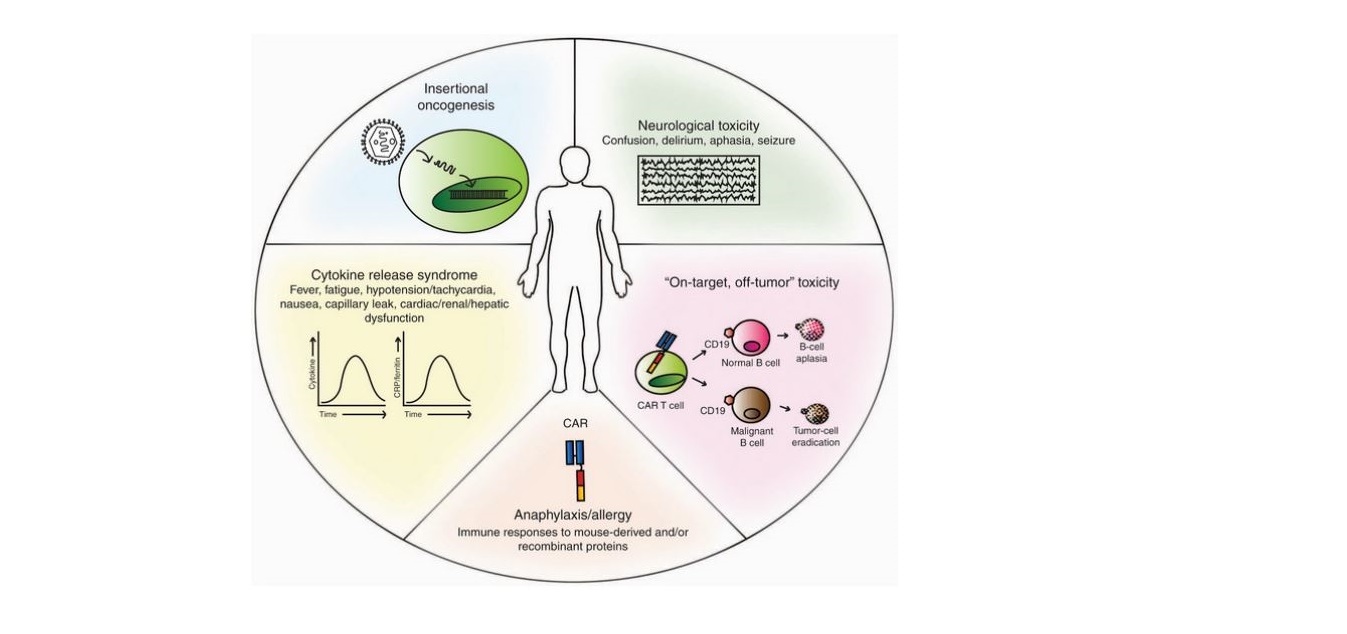
Figure 1. Toxicities of chimeric antigen receptor (CAR) T-cell therapy. Depiction of reported/potential toxicities following the use of CAR-T cells: insertional oncogenesis (theoretical); neurological toxicity (NTX); “on-target, off-tumor” toxicity (engagement of target antigen on nonpathogenic tissues); anaphylaxis/allergy (host reaction to foreign antigen expressed by the CAR-T cell); cytokine release syndrome (CRS) (systemic inflammatory response following activation of CAR-T cells). CRP, C-reactive protein. Used under CC BY-NC-ND 4.0
B cell aplasia and hypogammaglobulinemia
As previously reported, the second-generation CAR-T cells recognizing the CD19 antigen on malignant cells are now used in FDA-approved treatments. Unfortunately, this target antigen is also present in normal B lymphocytes, resulting in some cases in a significant decrease of their numbers and consequent reduction in the number of immunoglobulins. In these situations, regular administration of pooled immunoglobulin was required to prevent potential complications from lowered immunity.Other on-target/ off tumor side effects
It is worth mentioning that studies on CAR-T cells, targeting other antigens than CD19, have also caused various side effects in which the modified lymphocytes attacked healthy tissues. For example, a study of renal cell carcinoma treatment with CAR-T cells specific for carboxy-anhydrase-IX resulted in the development of cholestasis [16]. Similar on-target\off-tumor recognition was observed in colon cancer trials where CAR-T cells specific for carcinoembryonic antigen were attacking normal colonic tissue and caused severe transient colitis [17].Summary
The high therapeutic efficacy of autologous CAR-T cells often acts as a double-edged sword. Therefore, new solutions like, e.g. silencing of the CAR-T cells in a controlled manner are being explored in preclinical and clinical trials. In the next part of our CAR-T therapies blog series we will focus on the improvements that are necessary to reduce toxicity and increase efficacy of CAR-T cell therapies.Bibliography
[1] Hill JA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018 Jan 4;131(1):121-130. doi: 10.1182/blood-2017-07-793760.
[2] Park JH, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis. 2018 Aug 1;67(4):533-540. doi: 10.1093/cid/ciy152.
[3] Lee DW, et al. Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10;124(2):188-95. doi: 10.1182/blood-2014-05-552729. Erratum in: Blood. 2015 Aug 20;126(8):1048. Dosage error in article text. Erratum in: Blood. 2016 Sep 15;128(11):1533.
[4] Gust J, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017 Dec;7(12):1404-1419. doi: 10.1158/2159-8290.CD-17-0698.
[5] Lee DW, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019 Apr;25(4):625-638. doi: 10.1016/j.bbmt.2018.12.758.
[6] Neelapu SS, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017 Dec 28;377(26):2531-2544. doi: 10.1056/NEJMoa1707447.
[7] Schuster SJ, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019 Jan 3;380(1):45-56. doi: 10.1056/NEJMoa1804980.
[8] Maude SL, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018 Feb 1;378(5):439-448. doi: 10.1056/NEJMoa1709866.
[9] Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014 Feb 19;6(224):224ra25. doi: 10.1126/scitranslmed.3008226.
[10] Chou CK, Turtle CJ. Assessment and management of cytokine release syndrome and neurotoxicity following CD19 CAR-T cell therapy. Expert Opin Biol Ther. 2020 Jun;20(6):653-664. doi: 10.1080/14712598.2020.1729735.
[11] Gardner RA, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019 Dec 12;134(24):2149-2158. doi: 10.1182/blood.2019001463.
[12] Santomasso BD, et.all. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018 Aug;8(8):958-971. doi: 10.1158/2159-8290.CD-17-1319.
[13] Gust J, et all. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019 Jul;86(1):42-54. doi: 10.1002/ana.25502.
[14] Hunter BD, Jacobson CA. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J Natl Cancer Inst. 2019 Jul 1;111(7):646-654. doi: 10.1093/jnci/djz017.
[15] Si S, Teachey DT. Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Evidence to Date. Ther Clin Risk Manag. 2020 Aug 4;16:705-714. doi: 10.2147/TCRM.S223468.
[16] Lamers CH, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013 Apr;21(4):904-12. doi: 10.1038/mt.2013.17.
[17] Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011 Mar;19(3):620-6. doi: 10.1038/mt.2010.272.




