Over the past decade, morphological profiling, which initially emerged as a curiosity in academic labs, has rapidly matured into a translational technology that delivers tangible results in the drug discovery field. How can we use it for this purpose?
From startup biotechs to pharma giants, researchers are leveraging cell images to predict how compounds work, repurpose existing drugs, and flag toxic side effects early [1,2]. The approach is paying off: AI-driven phenotypic screening has already accelerated the development of drug candidates into clinical trials in record time [3].
In this article, we explore selected success stories that highlight the impact of morphological profiling throughout the drug discovery pipeline. We’ll also consider where in R&D this method adds the most value. The ability to comprehensively quantify changes in cell morphology at scale is transforming how we find and develop new medicines.
What Is Morphological Profiling and Why Does It Matter?
Morphological profiling (also known as image-based profiling or cytological profiling) is a powerful, unbiased approach that captures a holistic snapshot of cell state by analyzing changes in cell shape, size, and texture in response to a drug or genetic perturbation. With the advent of high-throughput automated imaging, the method has become a valuable tool in phenotypic drug discovery.
Instead of examining a single data point from a target-based assay, profiling casts a wide net, generating a rich fingerprint that characterizes the total impact of a chemical, genetic, or other perturbation at single cell resolution.
The leading method for this is the Cell Painting assay, developed in Anne Carpenter’s lab at the Broad Institute. Cell painting is a high-content image-based assay used for cytological profiling. It utilizes a set of fluorescent dyes to “paint” and visualize eight key cellular components and organelles, including the nucleus, endoplasmic reticulum, mitochondria, and Golgi apparatus. It generates thousands of morphological measurements per cell using automated image analysis software, depending on the tools and methods used [4].
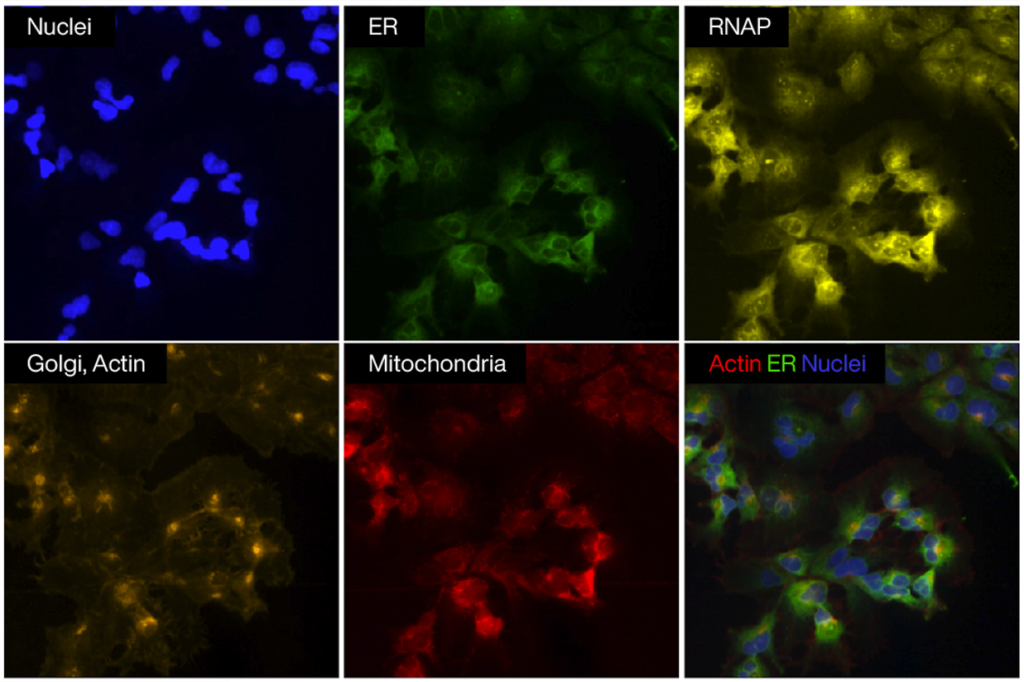
Figure 1. Fluorescence microscopy images showing individual cellular components
A key advantage is the speed and cost of morphological profiling data analysis workflows. A Cell Painting experiment can profile hundreds of thousands of compounds in parallel with a rich readout, whereas developing a comparable battery of targeted assays would take far longer. Because it’s a general morphological profiling assay, data from one screen can be repurposed for multiple questions – for instance, to predict various bioactivities or toxicities later [4,5].
Since its publication in 2016, the Cell Painting paper has been cited over 2,300 times (as of 2025), reflecting its rapid adoption across academia, biotechnology, and pharmaceutical industries. The method’s scalability, reproducibility, and versatility have made morphological profiling a cornerstone of AI-powered phenotypic drug discovery.
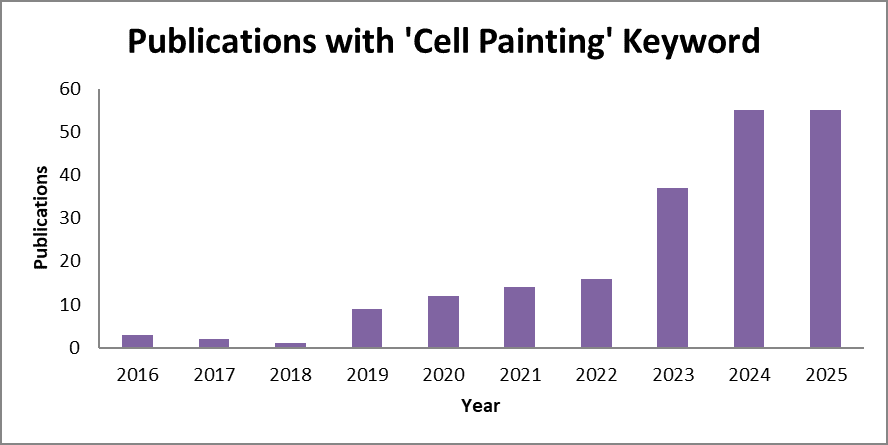
Figure 2. A bar chart presenting constant growth in the Cell Painting method citation, with over 50 publications published in each of the two recent years.
Cell Painting Assay Meets Deep Learning: Success Stories from Industry
At the same time, rapid progress in machine learning and deep learning, especially in computer-vision architectures, has transformed how effectively we extract signal from high-content images. The application of deep learning for morphological profiling turns image-based assays into a reliable, scalable source of actionable biological insight.
Beyond its methodological elegance, image-based profiling finds value at multiple stages of drug discovery: in hit identification, it enables unbiased phenotypic screening; in lead discovery and optimization, it detects off-target or toxic effects; and in translational research, it supports mode-of-action (MoA) annotation and compound repurposing.
Let’s take a closer look at the most notable examples of morphological profiling approaches in the pharmaceutical industry.
Drug Discovery From Screen to IND in 18 Months: Recursion Pharmaceuticals
Recursion Pharmaceuticals was the first company to stake its entire platform on morphological profiling. Co-founded in 2013 by two graduate students inspired by Anne Carpenter’s work, Recursion’s vision was to build a “map of connections among diseases, genes, and drugs using image data [6]. As Anne recalled in TechBio Talks, initially she was unsure how much impact the method could have until Recursion confirmed that phenomics could directly translate into clinical impact [7].
In 2024, Recursion received FDA clearance for its AI-discovered cancer drug REC-1245, reaching this stage in under 18 months [3]. Far faster than the multi-year industry norm.
REC-1245 targets RBM39, a protein similar to a difficult-to-drug cancer target (CDK12). Recursion’s algorithms pinpointed RBM39 and its potential role in multiple cancers based on morphological profiles [3]. By late 2024, the Phase 1/2 trial was underway, making REC-1245 one of the first AI-discovered molecules to reach patients [3].
Recursion initially cut its teeth on drug repurposing for rare diseases as morphological profiling was “successfully used to identify potential new uses of known drugs for treating cerebral cavernous malformation (CCM), a hereditary stroke syndrome” [4]. But Recursion’s pipeline has expanded to novel compounds.
Another compelling validation of Recursion’s repurposing approach is REC-4881, a molecule tested initially in solid tumors. Early Recursion OS identified MEK1/2 inhibition as a rescue mechanism for APC loss-of-function biology in familial adenomatous polyposis (FAP). REC-4881 emerged as a top phenotypic hit and was advanced as the first MEK1/2 inhibitor studied clinically for FAP, a disease with no approved therapies [8].
TUPELO phase 1b/2 trial data show meaningful and durable reductions in polyp burden. 75% of patients responded at 12 weeks and 82% maintained reductions 12 weeks after stopping treatment [8].
Recursion also developed the Phenom model, which can extract significant biological insights from simple brightfield images and nearly matches the performance of Cell Painting [9].
Recursion’s success shows how pairing morphological profiling with deep learning can shorten discovery timelines, expand therapeutic possibilities, and validate morphological profiling as a true engine of modern drug discovery.
Predicting Biological Activity and MoA: AstraZeneca
Morphological profiling is not used only by Recursion; pharmaceutical companies widely adopt it to leverage the massive data they have collected over the years in previous projects.
Researchers at AstraZeneca (AZ) trained deep learning models on 8,300 compounds, combining Cell Painting images with single-concentration bioactivity data from 140 assays. The models achieved strong performance (ROC-AUC 0.744 ± 0.108) and clearly outperformed feature-based approaches and chemistry-only predictions [10].
In several cases, adequate prediction could even be achieved using brightfield images alone, highlighting a potential path to lower experimental costs.
The method was especially effective for cell-based assays and kinase targets, yielding greater scaffold diversity among the top-ranked predictions. Experimental follow-up confirmed substantial enrichment of true actives: up to 14.76× for specific targets [10].
Together, these findings show that Cell Painting-image-based models, even with minimal activity data, can reliably prioritize active and structurally diverse compounds across a wide range of targets.
One of the AstraZeneca research groups integrated morphological profiling in MoA and safety studies of PROTACs (proteolysis-targeting chimeras). PROTACs are complex, bivalent molecules that degrade a protein of interest using the ubiquitin-proteasome system. As compounds that typically reside outside the traditional small-molecule drug physicochemical property space (known as bRo5 compounds), predicting their safety risks and off-target effects can be tricky.
AZ scientists applied Cell Painting to profile a series of PROTACs. They discovered a distinct mitochondrial toxicity signature that would not have been evident from looking at the PROTACs’ individual warhead or linker components [11].
This is a prime example of why unbiased profiling is essential: it can uncover emergent toxic liabilities that targeted, hypothesis-driven assays would inherently miss.
Flagging Toxicity Early: Bayer and Janssen
Safety-related attrition is a significant cost in pharma R&D, and morphological profiling is showing promise in predicting toxicity before a compound ever reaches animal or human testing. Specific cytological changes seen in Cell Painting images serve as early warning signals for toxicity.
For example, swollen mitochondria, condensed nuclei, or phospholipid accumulation in cell images may correlate with organ toxicities. Two recent industry studies combined imaging with machine learning to improve toxicity prediction.
Researchers at Bayer focused on mitochondrial toxicity, a common cause of drug-induced liver or heart injury. They developed machine-learning models to predict mitochondrial dysfunction, comparing models based on chemical structures alone versus those incorporating Cell Painting image features.
The result: “models incorporating morphological profiles perform better in predicting mitochondrial toxicity than those trained on chemical structures alone” [12].
In quantitative terms, adding Cell Painting data improved the predictive Matthews correlation coefficient (MCC) by ~0.08-0.09 (relative gains of 10-25% in some validation tests) [12]. The cell images provided orthogonal information that chemical structure alone couldn’t. The Bayer study concluded that an integrated approach is crucial for understanding cell biology in complex endpoints, such as mitochondrial disruption [12].
A perennial issue in predictive toxicology is that models often fail for novel chemotypes [13]. Scientists at Janssen (J&J) explored the use of Cell Painting to extend QSAR (quantitative structure-activity relationship) models into new chemical space.
Their 2023 paper showed that combining morphological image features with molecular structure features could “expand the applicability domain” of toxicity prediction models, making them more reliable for structurally novel compounds.
They demonstrated an active learning cycle, where compounds flagged by the image-based model were tested and added, resulting in a nearly 16% expansion of the model’s chemical space coverage and an almost 10% improvement in prediction accuracy [13]. This approach of leveraging phenotypic data as an adjunct to traditional toxicology assays is highly alluring, as it means fewer missed hazards and potentially fewer late-stage failures.
Overall, these success stories prove that distinct morphological profiling changes can serve as an early safety radar, detecting hints of organ toxicity (such as mitochondrial injury) in vitro and guiding chemists to steer clear of risky candidates sooner.
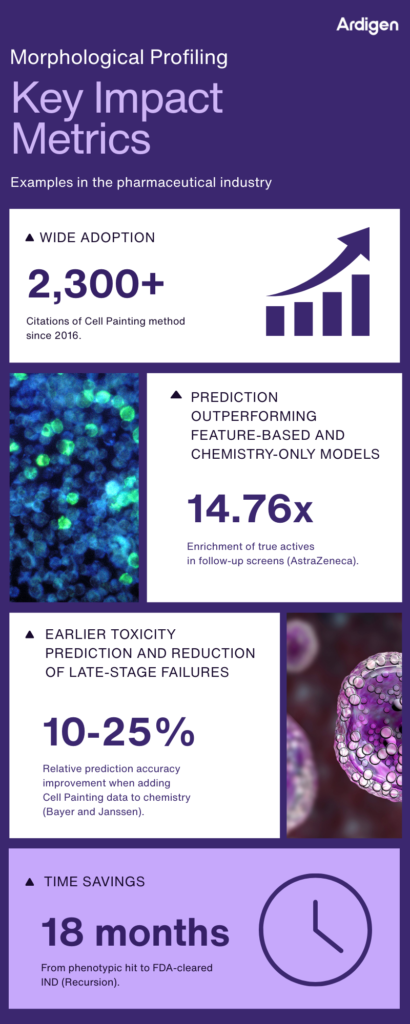
Figure 3. Key impact metrics illustrating how morphological profiling, combined with AI-driven image analysis, enhances compound prioritization, toxicity prediction, and development timelines across pharmaceutical R&D.
Ardigen Applications of Morphological Profiling
For biopharmaceutical organizations under pressure to shorten timelines and reduce the risk of early decisions, the return on investment from morphological profiling is a burning issue. At Ardigen, we see this every day.
Our current collaborations with global pharmaceutical leaders demonstrate how Ardigen phenAID doubles predictive accuracy, increases operational efficiency, and reduces analysis costs by more than 50%. Below, we present examples of such fruitful collaborations.
Case Study 1 – Predictive Power from Sparse, Heterogeneous Cell Painting Image Data
A global pharmaceutical partner approached Ardigen with a challenge common in large-scale phenotypic programs: they possessed an enormous Cell Painting image dataset, but the biological signal was sparse, labels were inconsistent across libraries, and batch effects masked the relationships they hoped to uncover. Traditional analytics struggled to extract usable insights, which slowed decision-making and eroded confidence in the data.
Ardigen phenAID platform delivered a step-change in value. By training custom AI models directly on raw images and expert-engineered morphological features, our team maximized signal recovery even when true biological activity was infrequent. We further harmonized datasets originating from multiple concentrations, across multiple cell lines, and screening libraries using batch-effect reduction techniques, building a unified and scientifically coherent dataset.
To support decision-making at scale, we embedded prediction reliability metrics, enabling scientists to immediately understand when a model’s output was trustworthy.
The impact was substantial:
- 2× improvement in accurate biological activity prediction.
- 20% increase in retrospective ROC AUC.
- 50% gain in loading and storage efficiency across the entire pipeline.
For the partner, this meant faster triage of compound candidates, more confident project directions, and far better utilization of existing imaging assets. In short: higher discovery ROI.
Read more on this case here: Extracting scientific insight from High Content Screening images – Ardigen | Top AI-Powered CRO for Drug Discovery & Clinical Trials.
Case Study 2 – Multimodal AI for Faster, More Accurate MoA and Bioactivity Prediction
Another client, a global pharmaceutical company, required enhancing the prediction of mode of action (MoA) and bioactivity using their Cell Painting dataset.
Ardigen’s team employed a multimodal machine learning approach that integrated morphological information from cell images with chemical descriptors and internal HTS data as labels. The Ardigen phenAID platform combined these inputs to train AI models capable of linking compound structure, phenotype, and biological response.
For the client, the ROI was immediate and quantifiable:
- Higher MoA and bioactivity prediction accuracy, enabling smarter compound triage.
- 4× faster pipeline compared to classical image analysis.
- 34× lower computational cost, dramatically reducing infrastructure spend.
- A reliable decision-support tool that identifies new compounds with desired phenotypic profiles.
This broad spectrum of analysis strategies resulted in a scalable, future-proof approach that strengthens portfolio decisions, increases hit-to-lead velocity, and reduces the cost per validated hypothesis.
Read more on this case here: Ardigen phenAID: Multimodal MoA & Bioactivity Prediction for HCS Dataset from a Big Pharma Company.
Looking Ahead: The Future of Morphological Profiling for Drug Discovery
Morphological profiling has moved from an academic concept to a proven component of modern drug discovery and continues to evolve. Recent success stories, such as AI-discovered clinical candidates, rare-disease repurposing wins, significant gains in hit rates and specificity, early toxicity alerts, along with widespread adoption across top pharmaceutical companies, clearly demonstrate that image-based profiling is a valuable tool.
Advances in deep learning are already enabling more nuanced image feature extraction (going beyond the ~1500 handcrafted features to automatic image representation learning) [14]. This could improve sensitivity to subtle phenotypes and enable cross-context predictions (e.g., predicting in vivo effects from in vitro images).
The massive JUMP-CP image set and other open resources will likely fuel the development of better models trained on cell painting images and transfer learning. This makes it easier to apply morphological profiling in new disease areas without having to start from scratch.
We also anticipate integration of morphological data with other data modalities, such as combining cell image profiles with high-throughput transcriptomics data (generated using Drug-seq technology developed by Alithea Genomics) or proteomics, to obtain a multi-modal view of drug action. Early studies suggest that morphology and gene expression capture overlapping but also distinct information [15] – together, they could provide very high predictive power for complex outcomes.
As the method becomes more routine, we may see regulatory agencies take an interest in it, supporting its role in drug discovery. Perhaps morphological profiling could be used in toxicity packages for IND submissions in the future to bolster evidence of safety (or at least to explain mechanisms of toxicity).
So let’s cast a wide net with Cell Painting and let pattern-recognition AI find hits and mechanisms from images and multimodal data without preconceived biases. As Anne Carpenter reflected, “Images as a readout are extremely flexible in the kinds of things we can detect (…). There aren’t many aspects of biology that can’t be captured using imagery” [2]. With morphological profiling, we are literally seeing new possibilities unfold. The technology’s trajectory suggests that its most impactful chapters are yet to be written.
We also encourage you to learn more about our morphological profiling platform – Ardigen phenAID.
Author: Martyna Piotrowska
Technical editing: Ardigen expert: Magdalena Otrocka, PhD
Bibliography
- Way GP, Sailem H, Shave S, et al. Evolution and impact of high content imaging. SLAS Discovery. 2023; 28(7), 292-305. https://www.doi.org/10.1016/j.slasd.2023.08.009
- Sakai J. A marriage of microscopy and machine learning [Internet]. Broad Institute. 2020 [cited 2025 Nov 5]. [Available from:] https://www.broadinstitute.org/news/marriage-microscopy-and-machine-learning
- Kasianov R. Recursion Pharmaceuticals receives FDA clearance for AI-discovered cancer drug trial [Internet]. BiopharmaTrend. 2024 [cited 2025 Nov 5]. [Available from:] https://www.biopharmatrend.com/news/recursion-pharmaceuticals-receives-fda-clearance-for-ai-discovered-cancer-drug-trial-977
- Bray MA, Singh S, Han H, et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat Proto. 2016;11:1757-74. https://doi.org/10.1038/nprot.2016.105
- Bray MA, Gustafsdottir SM, Rohban MH, et al. A dataset of images and morphological profiles of 30,000 small-molecule treatments using the Cell Painting assay. Gigascience. 2017;6(12):1-5. https://doi.org/10.1093/gigascience/giw014
- Anne Carpenter’s vision for Recursion [Internet]. Recursion. [cited 2025 Nov 5]. [Available from:] https://www.recursion.com/news/painting-the-picture-of-recursion-anne-carpenter-scientific-advisory-board-member
- Carpenter A, Gibson C. Broad Institute’s Anne Carpenter with Host Chris Gibson [Internet]. TechBio Talks. 2025 [cited 2025 Nov 5]. [Available from:] https://open.spotify.com/episode/45jKbnnyqIqOr0vdd34RUQ
- Positive Phase 1b/2 Results from Ongoing REC-4881 TUPELO Trial Demonstrate Rapid and Durable Reductions in Polyp Burden in Familial Adenomatous Polyposis (FAP) at 25 Weeks [Internet]. Recursion. [cited 2012 Dec 9]. [Available from:] https://ir.recursion.com/news-releases/news-release-details/positive-phase-1b2-results-ongoing-rec-4881-tupelo-trial-0
- Kraus O, Kenyon-Dean K, Saberian S, et al. Masked Autoencoders for Microscopy are Scalable Learners of Cellular Biology. Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR). 2024 [cited 2025 Dec 4]:11757-11768. [Available from:] https://openaccess.thecvf.com/content/CVPR2024/papers/Kraus_Masked_Autoencoders_for_Microscopy_are_Scalable_Learners_of_Cellular_Biology_CVPR_2024_paper.pdf
- Fredin Haslum J, Lardeau C-H, et al. Cell Painting-based bioactivity prediction boosts high-throughput screening hit-rates and compound diversity. Nat Commun. 2024;15(1):3470. https://doi.org/10.1038/s41467-024-47171-1
- Trapotsi M-A, Mouchet E, Williams G, et al. Cell morphological profiling enables high-throughput screening for PROteolysis TArgeting chimera (PROTAC) phenotypic signature. ACS Chem Biol. 2022;17(7):1733-44. http://dx.doi.org/10.1021/acschembio.2c00076
- Garcia de Lomana M, Zapata P A M, Montanari F. Predicting the Mitochondrial Toxicity of Small Molecules: Insights from Mechanistic Assays and Cell Painting Data. Chemical Research in Toxicology. 2023;36(7):1107-20. https://doi.org/10.1021/acs.chemrestox.3c00086
- Herman D, Kańduła MM, Freitas LGA, et al. Leveraging Cell Painting Images to Expand the Applicability Domain and Actively Improve Deep Learning Quantitative Structure – Activity Relationship Models. Chemical Research in Toxicology. 2023;36(7):1028–36. https://doi.org/10.1021/acs.chemrestox.2c00404
- Krentzel D, Shorte SL, Zimmer C. Deep learning in image-based phenotypic drug discovery. Trends Cell Biol. 2023;33(7):538–54. https://dx.doi.org/10.1016/j.tcb.2022.11.011
- Way GP, Natoli T, Adeboye A, et al. Morphology and gene expression profiling provide complementary information for mapping cell state. Cell Syst. 2022;13(11):911-923.e9. https://dx.doi.org/10.1016/j.cels.2022.10.001